GAS CHROMATOGRAPHY | BASIC PRINCIPLE, CONSTRUCTION, AND APPLICATIONS OF GAS CHROMATOGRAPHY GC | COMMON DETECTORS USED IN GC
What is Gas Chromatography or GC?
Chromatography is a separation technique. Gas chromatography is an instrumental technique used for the separation of a mixture of components. In the gas chromatography technique, the mobile phase is gas and the stationary phase is liquid or solid. When the stationary phase is solid it is known as Gas-Solid chromatography. (G.S.C.) and when the stationary phase is liquid, it is known as Gas-Liquid chromatography – (G.L.C).
In the Gas Chromatography process, the sample is converted into gas form if it is not initially gas form by heating separately in heating blocks. This gaseous form of the sample is then mixed with the inert gases which may be N2, H2, He, or Ar. But H2 is avoided as it is highly explosive. The carrier gas and compartment of the sample are then injected into the instrumentation which contains a stationary phase either solid or liquid. The component of the sample will be retained at the different positions in the stationary phase.
Pass the suitable eluent as a carrier gas. Less strongly retained is eluted out first and the more strongly retained component is eluted out last.
The eluted out components are the incident on the recorder and detector. The tR helps us in qualitative analysis. The number of the peaks corresponds to the number of components present in the mixture. The area of each peak indicates the amount of that component present in the given mixture means qualitative analysis.
It means with the help of Gas Chromatography qualitative, quantitative, and separation of the mixture is also possible.
How does the Gas Chromatography or GC work?
Gas Chromatography Instrument

Components used in Gas Chromatography-
i) Carrier gas tank:- (Cylinder)
In this tank carrier gas for e.g.:- He, N2, Ar, or H2 is present under high pressure. The carrier should be inert, non-reactive, and available in a pure state. A carrier gas is selected on basis of the detector used.
ii) Flow regulator or controller:-
The flow of the carrier gas is controlled, as it is attached to the carrier gas tank. The pressure usually maintained is 10-50 pounds per square inch with a flow rate of 25-40 ml min-1.
iii) Sample injection unit:-
The sample is injected through a silicon rubber septum. The sample size is of order 0.01 to 10 µdm3over sized sample causes broadening of peak. The temperature of the unit is maintained at 60º C above the normal boiling point of the sample.
iv) Separation column:-
The columns and packing used in Gas-Liquid Chromatography are
i) packed column and ii) capillary column.
Capillary column is coated with a very thin film of liquid stationary phase, they are of length 100-300 meters, Theoretical plates are of tune-up to several hundred thousand. Packed columns of glass or metal tubes of 1-8 mm diameter and 2-20 m in length are coiled and a number of theoretical plates are about 20,000 or more.
Ideal supports to be used for the liquid stationary phase should be
i) chemically inert ii) posses mechanical strength to withstand pressure iii)large surface area iv)known pore size.
Most commonly used solid support Firebrick and Kieselguhr
Ideal liquid to be used as liquid stationary phase should be
i) Chemically inert and thermally stable ii) Low or negligible vapour pressure iii) boiling point should be 200ºC more than operating temp of instrument iv) solvent characteristic.
Commonly used liquids are silicone oil or grease. Polyethylene glycol for polar sample. Benzyl dipyridil for aromatic sample. etc
The columns and packing used in the case of Gas-Solid Chromatography
Solid adsorbents are used as stationary phases in the column. For eg Carbon in different varieties is used. Zeolites have defined open structures with holes, hence used for separation of the sample depending upon the size of the sample. Ion exchange resin and some complex inorganic salts can also be used in a few cases.
v) Detector used in Gas chromatography GC:-
It is the device present at the exit of the column. It measures the amount eluted out by carrier gas. The temperature of the detector component must be high to prevent
the condensation of the sample vapours and at the same time, the sample components should not be decomposed. The commonly used detectors are —
- Thermal conductivity detectors (T.C.D.)
- Flame ionization detectors (F. I.D.)
- Electron capture detectors (E.C.D.)
vi) Signal Recorders:-
The output of the detector is fed into the signal recorder. The results of the signal are recorders give qualitative and quantitative analysis.
vii) Thermostat:-
The sample injection components’ chromatographic column and detectors must be constant and the components must be in the gaseous form.
Working of Gas chromatography
Suppose the mixture contains three-component A, B& C. If this mixture is liquid then it is to be preheated in the heating blocks, so that it must be converted into a gaseous form. At the same time, the carrier gas is allowed to flow with pressure from the carrier gas tank. The flow of the carrier gas is to be regulated by the flow regulator and is then mixed with the sample vapour with the help of a rubber septum by syringe.
It is injected into the head of the column. As the mobile phase move from one end to the other end retention or adsorption takes place on the stationary phase which may be solid or liquid. The gas which comes out from the column is only carrier gas.
Now, we have to pass another carrier gas which is known as eluent. The least retained component is eluted at first. Suppose C will be eluted first and last will be A. If we plot a graph of signal recorder vs. time. The graph is consist of peaks. The no. of peaks corresponds to no. of the components. From the retention time, tR. We can identify the component. The concentration of each component is the area of each peak.
Gas Chromatograph
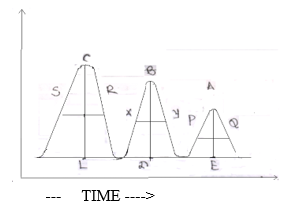
- Conc. of C = area of peak
C = l(CL) x l( SR)
- Conc. of B = area of peak B
=l(BO) x l( XY)
- Conc. of A =area of peak A
=l(AE) x l( PQ)
Applications of gc Chromatography or Uses of Gas chromatography
Applications of gc chromatography are as follows.
a. The black smoke which comes out from the vehicles due to the burning of fuel is commonly called auto exhaust gases. These auto exhaust gases can be analyzed by Gas chromatography & thus pollution caused by these gases can be studied.
b. The B.P. of benzene is 80.1 0c & cyclohexane is 80.80c. If we have the mixture of two which are miscible then by using any efficient distillation kit we can not separate the miscible mixture as the difference in the boiling point is just 0.70c. The mixture of the two can be separated & analyzed by Gas Chromatography.
c. Radioactive products can be analyzed by Gas Chromatography.
d. Suppose we have the mixture of H2, CO2, CO, CH4, C2H6, C2H4, C2H2, etc., which can be separated by using the molecular sieves.
e. Reaction mechanism can be studied with the help of Gas Chromatography.
f. The content of ethyl alcohol present in the blood can be determined with the high accuracy using Gas Chromatography
g. With the help of gas chromatography, we can analyze the body fluids within a few minutes which may result in the complete diagnosis of the body.
h. The mixture of primary, Secondary & tertiary amines can be separated by mixing the mixture with 2, 4 – dinitrofluoro benzene in Gas Chromatography.
i. Natural products can be analyzed and new compounds can be synthesized.
j. Purity of the metals can be tested. The presence of Al and Cr in uranium can be tested.
k Separation of hundreds of hydrocarbons in petroleum is possible by Gas-liquid chromatography.
Detectors used in Gas Chromatography
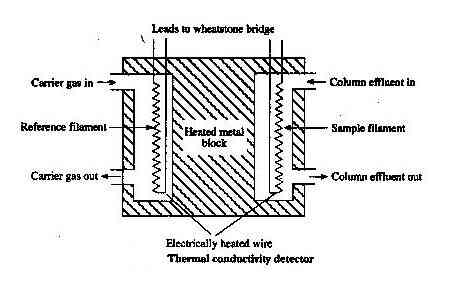
Thermal Conductivity Detector (T.C.D.)
It is also known as a katharometer.
Principle
The rate of the cross of heat from the body depends on the thermal conductivity of the surrounding gas. The thermal conductivity of the surrounding gas depends on its composition. Thus the rate of heat depends on the composition of the surrounding gas.
Construction
It contains two identical brass cells. The cells contain fine platinum wire. Effluent from the column flows through one cell and carrier gas through another cell. Wires are heated by the current through the accumulator.
As long as there is no sample gas the resistance of the wire will be the same but whenever the sample component is eluted with carrier gas there is heat loss which unbalances the bridge & gives rise to deflection in galvanometers. The signal on amplification causes the pen to move on a strip of moving graph.
The thermal conductivity of helium & hydrogen is found to be ten times more than that of most organic compounds. Hence the introduction of organic compounds brings a sharp change in conductivity.
The detector is simple inexpensive & accurate but it is not extremely sensitive to another gas.
Flame Ionisation Detector (F.I.D.)
Construction of flame ionization detector (FID)
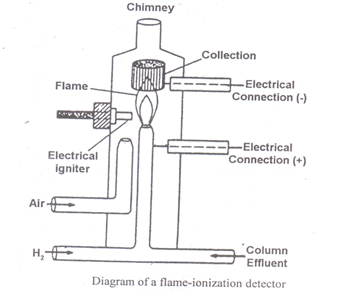
When an organic compound is burnt in an air–hydrogen flame at the temperature of the flame, ions are produced. These ions produced in the flame are collected and the resulting ion current is measured Magnitude of the ion current produced is proportional to the no. of carbon atoms reduced.
Even though the response is proportional to the no. of carbon atoms entering the flame those which are already completely ionized do not respond to ion formation. The detector is insensitive to most the inorganic compounds including air and water.
When the effluent enters the burner base and it mixes with hydrogen and air and is burned at the HP of the jet. The jet forms the negative electrode and the collector electrode from the positive electrode. A potential of 400 V is applied across the two electrodes. This lowers the resistance across the gap between the two electrodes and causes a current to flow.
The temp range is kept between 10 and 420oc when only pure carrier gas has been introduced to the flame, due to negligible ionization low current levels are observed. Most of the organic compounds are from ions in the flame
when the carrier gas is mixed with the compound molecule extent of ionization increases. The ion current increases which after amplification is fed to the recorder.
Electron capture detectors (E.C.D.)
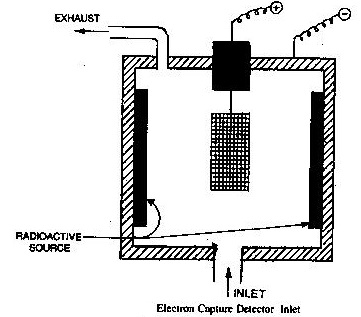
It works on the principle that the carrier gas can be ionized by means of a radioactive source into electrons. The ionization involves the formation of the ion pair. These electrons move towards an anode under a fixed voltage. This constitutes a steady current when only carrier gas is present.
When carrier gas is mixed with an electron capturing molecule of sample in the detector it absorbs some electrons which results in the reduction in no. of electrons and cause a drop in the standing current. The drop in current is directly proportional to the concentration of components in the sample. The drop in the current is amplified & recorded.
ECD is insensitive to amine, alcohols, and hydrocarbons but is very sensitive to halogen anhydride, and nitrile. Organometallic which has an affinity for electrons.
The column is made up of a variety of materials including glass metal such as copper, Stainless steel or it may be made up of organic polymer. The length of the tube varies from a few cm to 100 meters. The internal diameter of the tube is 4mm. The column is in the form of a spiral and is filled with silica gel or alumina or activated carbon or molecular sieves are used for G.S.C.
For G.L.C. the column is filled with a mixture of ethers and esters or hydrocarbons of high molecular weight. These liquids are supported by elite or glass beds in some cases. Greases and silicone oils can be used as a stationary phase.
What is Retention time in Gas Chromatography? How to calculate retention time in Gas Chromatography?
It is defined as the time taken by the component to move over the entire length of the column and hence to come out of it. Retention time depends on the nature of the stationary phase, mobile phase, flow rate, and column temperature chemical constitution.
Under a given set of conditions, retention time is characteristic of a given compound.
It is also used for the identification purpose. VR = tRF
Where VR = Retention volume, tR = Retention time, and F = Volume rate of flow of mobile phase.
Retention Volume: – [VR ]
Retention Volume is defined as the total volume of the mobile phase required to elute the component completely from the column. It is the characteristic of the component. It is given by equation.
VR = Vm+ K Vs
Where VR = Retention Volume
Vm = Volume of mobile phase
Vs = Volume of stationary phase
K = Constant.
When the solute peak maximum appears at the column exit, one-half of the total solute has eluted in retention volume while the other half remains in the mobile and stationary phase in the column.
Retention Ratio
Retention Ratio is the fraction of the total time spared by the solute in the mobile phase. If tm is time spared by solute in mobile phase ant ts is time spent in stationary phase then Retention ratio.
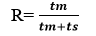
It is characteristic of compounds and hence used in identification purposes.
Peak Resolution :
Peak resolution is a measure of the extent of overlap between two adjacent components. It is a measure of the success of a given separation.
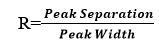
tR1ad tR2are retention times for components 1 & 2 respectively and W1&W2 are peaks at the base. For a good separation values of R should be greater than 1.5
What is Height equivalent to a theoretical plate (HETP) in Gas Chromatography?
The efficiency of a column depends on the number of theoretical plates that the column is supposed to be made up of. The greater the number of theoretical plates grater will be the efficiency of the column. The HETP for the column will be given as –
H = L / b
Where L – length of the column. b – no. of theoretical plates.
Therefore H = Lw2 / 16 + R2
Where tR – Retention time.
What elutes first in Gas Chromatography?
Less strongly retained is eluted out first and the more strongly retained component is eluted out last
When to use Gas Chromatography?
For qualitative, quantitative, and separation of the mixture.
What is GC? or What is the full form of GC?
It is Gas Chromatography.
What does gas chromatography separate based on?
In Gas Chromatography separation was observed based on differences in boiling points and polarity of components.
Thanks on your marvelous posting! I definitely enjoyed reading it, you will be a great author.I will be sure to bookmark your blog and will eventually come back in the foreseeable future. I want to encourage continue your great writing, have a nice afternoon!
Very interesting information!Perfect just what I was searching for! “Peace, commerce and honest friendship with all nations entangling alliances with none.” by Thomas Jefferson.
I¦ve been exploring for a little bit for any high-quality articles or blog posts on this kind of area . Exploring in Yahoo I finally stumbled upon this web site. Reading this information So i am glad to show that I have an incredibly excellent uncanny feeling I found out exactly what I needed. I such a lot surely will make certain to don¦t put out of your mind this web site and provides it a look regularly.
I am not certain the place you’re getting your info, but great topic. I must spend a while finding out more or understanding more. Thanks for fantastic information I was looking for this info for my mission.
I think other website owners should take this site as an model, very clean and wonderful user pleasant pattern.
Thank you very much dear
I was examining some of your blog posts on this internet site and I conceive this web site is really instructive! Continue putting up.
Some truly nice and useful information on this website, also I conceive the layout contains excellent features.
I am glad that I found this web site, just the right information that I was looking for! .
I want to express my love for your kind-heartedness giving support to persons that need assistance with this important area of interest. Your real dedication to passing the message all-around turned out to be quite advantageous and has regularly empowered those much like me to realize their desired goals. Your personal valuable advice implies this much a person like me and a whole lot more to my fellow workers. Best wishes; from all of us.