TREATMENT AND PRESENTATION OF ANALYTICAL DATA | ERRORS, ACCURACY AND PRECISION
The accuracy of a single measurement or that of sets of measurements is inversely related to the magnitude of error involved. Error is…
Simplified knowledge

The accuracy of a single measurement or that of sets of measurements is inversely related to the magnitude of error involved. Error is…
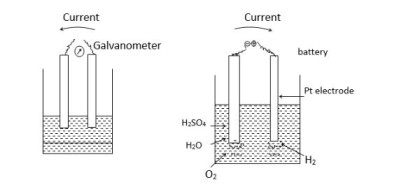
What is Polarization? Polarization The disturbance of equilibrium associated with the passage of current is called electrolytic polarisation and the electrode thus disturbed…

Principle of atomic absorption spectroscopy (AAS) There are two main types of Atomic spectroscopy namely Atomic emission spectroscopy- Flame emission spectroscopy (Flame photometry) and Atomic absorption spectroscopy…
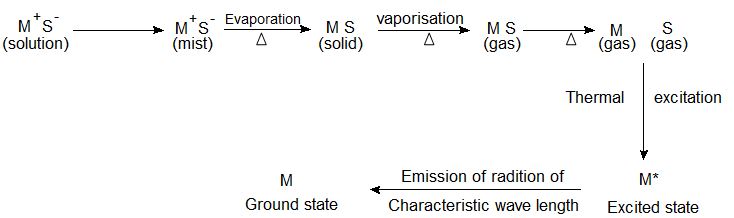
Optical methods of analysis are non-destructive techniques for testing. Optical methods for analysis are based on either absorbance or fluorescence. There are two…

BASIC INFORMATION ABOUT SALMON FISH Salmon is the very common name for this species of fish that belongs to a ray-finned family named…

BASIC INFORMATION ABOUT POMFRET Pomfret is one of the most popular species of fish like Salmon and Catla fish and is very commonly…

BASIC INFORMATION ABOUT CATLA FISH (KATLA FISH) Catla fish is also known for its fast growth and for the most important aquacultured fish…

Introduction - Surface Chemistry Surface chemistry is the study of the phenomena that take place on the surfaces of substances like adsorption, colloids, etc.…

Basic information about tuna fish A tuna is a fish that mostly lives in saltwater. It belongs to the tribe of thunnini which…

ABOUT CATFISH A Catfish is a diverse group of ray-finned fish. The name catfish is due to their probable, Which resembles cats' whiskers.…