Applied Electrochemistry | Polarization, Decomposition potential and over voltage in electrochemistry | Over voltage protection
What is Polarization?
The disturbance of equilibrium associated with the passage of current is called electrolytic polarisation and the electrode thus disturbed is said to be polarized. Polarization is supposed to be due to an irreversible behavior of an electrode. The irreversibility arises as a result of the slowness of one or more of the processes taking place at the electrode during the formation or discharge of an ion. The nature of the slow process decides the type of polarization.
Concentration Polarization & Causes of Polarization
Polarization can be due to,
(i) Slowness of one or other stages in the electrode process.
(ii) Slowness of the diffusion of ions in solution.
Polarization due to slowness of the diffusion of ions in solution results from concentration changes taking place in the vicinity of an electrode during electrolysis and is known as “Concentration Polarization”.
E.g. At an anode, the dissolution of the metal increases the number of ions in contact with it in the solution and if diffusion is slow, the concentration of cations in the immediate vicinity of anode will be greater than that in the bulk of the solution.
The phenomenon of polarization. How polarization can be eliminated.
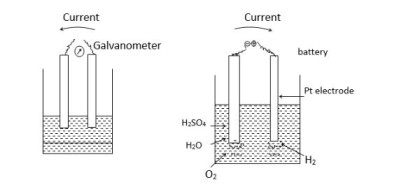
When the electric current is passed between Pt electrodes which are placed in dil H2SO4. H2 and O2 gases are liberated at the cathode and anode respectively.
a] Outside the solution, the current flows from the cathode (-) to the anode (+), while inside the solution it flows from the anode to the cathode.
b] If the battery is removed and the two electrodes are connected with a galvanometer, a small current will be seen to flow between the two electrodes. The direction of the current will be seen to flow opposite to theirs during the electrolysis.
c] It so happens because during the electrolysis ‘Pt’ electrodes get covered with the bubbles of H2 and H2 which constitute the gas electrodes and two will form the electrochemical cell. It has some fixed value of E.M.F. This phenomenon of back emf brought by the products of the electrolysis is known as ‘polarization’.
d] It is the difference in potential that is practically found and calculated with the help of the Nerst equation.
e] This polarisation is due to concentration changes in the vicinity of electrodes. Therefore It is commonly called concentration polarisation.
The factors which can minimize the polarization.
1. If the electrolyte is constantly stirred whole the bulk will have the same concentration. There is no increase in concentration in the vicinity of electrodes and polarisation does not take place.
2. To remove the deposition of gases that form the gas electrodes are brushed from time to time.
3. The deposition of the gases on the surface of electrodes is minimized by applying black platinum chloride PtCl4 on the surface of Pt electrodes and the electrodes are known as platinized Pt electrodes.
4] The polarization phenomenon can be eliminated by adding a strong oxidizing agent e.g. ‘HNO3’. Such substances which minimize the polarisation are called ‘depolarisers’.
What is meant by ‘Decomposition potential’ and how it can be measured experimentally?
Decomposition Potential – The minimum external voltage that must be applied to an electrolyte cell to bring about smooth continuous electrolysis is known as ‘decomposition potential (Ed).
Ed = Ec + Ea
Where, Ec = Cation
Ea = anion
Experimental Method to verify Decomposition potential
It consists of a cell, two electrodes are immersed in the electrolyte. Two electrodes are connected with a voltmeter through a galvanometer. The battery and variable resistance are connected across two electrodes.
Construction setup for Decomposition potential
The setup consists of the:-
a] External Source:- That can provide the required potential potentiometer through which the potential can be applied.
b] The electrolyte is under study, with two electrodes and a stirrer. The stirrer is used to stir the solution continuously.
c] The electrodes are connected to the variable resistance and external source of e.m.f. through Ammeter.
d] The variable resistance is used to change the applied voltage applied to the cell.
e] The voltage is measured by a voltmeter across the electrode.
f] An ammeter is used to measure the current through the circuit for any applied potential.

Working of Decomposition potential
a] In the beginning small potential is applied and the current flowing through the cell is measured through an ammeter.
b] The applied potential is gradually increased and the current flows.
c] For each value of applied voltage is plotted and the decomposition potential of the electrolyte is determined from the graph.
i) Along the line AB, electrolysis does not occur. The current is in accordance with Ohm’s law and is nonfaradic in nature.
ii) The curve BD represents the change in the current below decomposition potential.
iii) The curve DE represents the change in the current above decomposition potential.
iv)The potential corresponding to point C is the decomposition potential.
Factors that affect Decomposition potential
i) ED depends on the nature of the electrolyte.
ii) Concentration of electrolytes.
iii) Nature of electrode.
iv) Nature of the product of the electrolysis.
v) Temperature.
Applications of Decomposition Potential
i) It is characteristic properties.
ii) As the Ed for Fe is more and for Cu, it is less, therefore they can be separated from the mixture.
Overvoltage – how overvoltage can be measured experimentally?
Over Voltage -The difference between the potential of the electrolyte at which gas evolution occurs and the theoretical reversible potential for the same is known as overvoltage or overpotential.
Factors affecting overvoltage
1. Current density
Overvoltage increases with the increase in current density.
2. Temperature
Overvoltage increases with a decrease in temperature.
3. Surface area of electrons
Overvoltage decreases with an increase in the surface area of electrons.
4. Pressure
At high pressure, overvoltage slightly decreases and at low pressure, it increases rapidly.
η = Ed – Er
Where,
η = overvoltage
Ed = decomposition potential
Er = Theoretical reversible potential
Experimental determination for overvoltage
Construction
The electrolytic cell contains two electrodes, an electrolyte, and a stirrer. The stirrer is used to stir the solution continuously. The stirrer is used to stir the solution continuously. The two electrodes are connected to the variable resistance and external source of EMF through an ammeter. The variable resistance is used to change the applied voltage to the cell
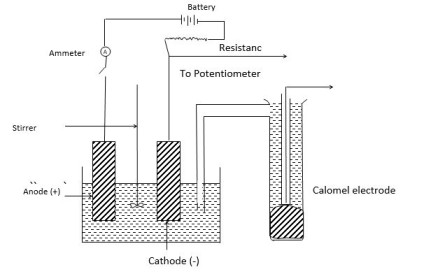
Determination of Overvoltage
Working
Using the source of potential and the variable resistance, the current density is maintained at the desired level. By moving the jockey over variable resistance, we have to keep the constant current.
Now the electrode and SCE are connected to a potentiometer and we determine the emf of the cell.
Ecell =Eelect + ESCE
For this, we can calculate the potential of the electrode. This potential of electrode we have determined practically so-called Ed. Now, we will write the electrode reaction and by applying the Nerst equation we will calculate the potential of the electrode. It is expressed as,
Overvoltage = Decomposition potential – Reversible electrode potential
η = Ed – Er
Over-voltage protection
Overvoltage protection is a power supply feature that shuts down the supply or clamps the output when the voltage exceeds a preset level.
At the cathode, the discharge of cations leads to a decrease in the concentration of cations in the immediate vicinity of the cathode. The anodic cathodic potentials will therefore be different from that calculated using bulk concentration. The concentration polarization is however of small magnitude. It can be readily eliminated by stirring the solution which will eliminate the concentration gradient and hence the concentration of metal ions at the electrode surface and in the bulk of the solution will be the same.
Tafel’s theory of Hydrogen Overvoltage
In 1905, Tafel defined the Hydrogen Overvoltage.
Hydrogen overvoltage is defined as, the potential in excess of than reversible potential required to discharge hydrogen ions at the surface of the cathode. Hydrogen overvoltage depends on
i) Current density
ii) Chemical nature of electrodes.
For the discharge of hydrogen ions at the cathode during electrolysis, the following steps are involved
i) Diffusion of hydrogen ions at the cathode.
ii) Transfer of diffused ion to the cathode surface.
iii) Discharge of hydrogen ions to hydrogen atoms.
iv) Adsorption of hydrogen atoms on the cathode surface.
v) Combination of the adsorbed hydrogen atoms to form hydrogen molecules.
vi) Evolution of hydrogen molecules as gas bubbles.
If any one of the above steps is slow, the electrode will not behave in a reversible manner.
According to Tafels, the adsorption of hydrogen atoms on the cathode surface is slow, which results in a high overvoltage Hydrogen atoms (adsorbed) to form hydrogen molecules is slow at high overvoltage.
The variation in hydrogen overvoltage (η) with current density (I) at constant temperature is given by the Tafels equation which is,
η = a + b log 𝒾
where, η = overvoltage
a = constant but varies from electrode to electrode.
b = value constant
𝒾 = current density
If we plot a graph of η vs log i, we get the value of b.

Electrodeposition or Electroplating
Electroplating
It is a process by which a coating metal is deposited on the base metal or alloy by passing a direct current through an electrolytic solution containing the soluble salt of the coating metal.
i) It is used to change the surface properties of metals as well as non-metals.
ii) It improves the appearance of base metals.
iii) The electroplated metals thus become resistant to corrosion, chemical attack, and water.
iv) It increases the hardness of the base metal.
Formula
Ed = Ec +Ea
Where, Ed = decomposition potential
Ec = cation & Ea = anion
η = Ed – Er
Where, η = overvoltage
Ed = decomposition potential & Er = Theoretical reversible potential
Ecell = Eelect+ ESCE
Er = 0.592 pH where (pH = – log H+ ions)
The objective of electroplating
(i) For decoration or to improve aesthetic qualities e.g. gold and silver coating on jewelry made of inferior metals.
(ii) For protection or corrosion protection e.g. chrome plating of metal parts in automobiles to enhance corrosion resistance.
(iii) For repair or build-up thickness on underside parts.
e.g. Welding of broken parts of machinery.
Thus, electroplating is used for depositing a layer of material to bestow a desired property to a surface that otherwise lacks that property. Some of the technological areas where electroplating methods are indispensable include all aspects of electronics, optics, sensors, etc.
Electroplating
Electroplating is a process by which a coating metal is deposited on the base metal by passing a direct current through an electrolytic solution containing the soluble salt of the coating metal. The base metal to be coated or electroplated is made cathode of the electrolytic cell and the coating metal is made anode.
Process of Electroplating
- It is carried out in electrolytic cells.
- The article to be electroplated is cleaned with organic solvents to make it free of oils, greases, etc.
- The clean article is then made cathode of the electrolytic cell.
- The anode is a coating metal.
- The electrolyte is a solution of coating metal.
- The anode and cathode are dipped in the electrolytic solution and a direct current is passed.
- The coating metal ions migrate towards the cathode and get deposited on the cathode and the pure anode oxidizes (dissolves) in the solution.
Theory of Electroplating
- A metal salt in an aqueous solution undergoes ionization.
- When a potential difference is applied to this salt solution through the electrodes, the metal ions migrate to the cathode and get deposited there.
- If the anode is of the same metal of which the salt is in the solution, the salt is reformed by the anode material passing into the solution in the form of ions.
- Hence the concentration of electrolytes remains unchanged.
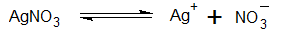
- For example, when silver nitrate solution is used as an electrolyte and silver is used as an anode or coating metal, the AgNO3 will ionize as,
6. On passing current Ag+ ions will migrate toward the cathode and get deposited on the article to be electroplated.

The equivalent number of Ag+ ions gets dissolved in the solution from the anode.
Purpose of electroplating
- To protect the metal from corrosion.
- To increase the commercial value of the metal.
What is decomposition potential in analytical chemistry?
The minimum external voltage that must be applied to an electrolyte cell to bring about smooth continuous electrolysis is known as ‘decomposition potential (Ed). Decomposition potential is inversely proportional to the temperature.
Application of Electrolysis?
Electrolysis is mainly used in the extraction and purification of metals