What is the Principle of Spectroscopy?
Spectroscopy is the study of the interaction between matter and radiation. When the light has been absorbed by the molecule there is some change in the behaviors of the molecule observed. These behaviors and their effects are going to study in this blog. We will also learn some basic concepts relevant to this.
The word spectroscopy is derived from two different words: spectrum is a Latin word it means image and skopia is a Greek word that means observation.
Some Basic concepts–
Dipole Moment
Dipole Moment:-Every molecule is composed of positively charged nuclei and negatively charged electrons in such a way that the molecule as a whole is electrically neutral.
It is “the product of the magnitude of charge (positive or negative) and the distance between them (bond length). If positive charge + q is separated from a negative charge –q by a distance ‘r’ then the dipole moment it is given by
µ = q x r Where µ – Dipole moment, q- Electronic charge, r-Bond length
Dipole moment arises due to the formation of a bond between two atoms of different electronegativity where the charge distribution is not uniform e.g. HC1. The CGS unit for dipole moment is the Debye, symbolized by D.
Bond Moment:- Any bond which has a degree of polarity has a dipole moment. This is called a bond moment.
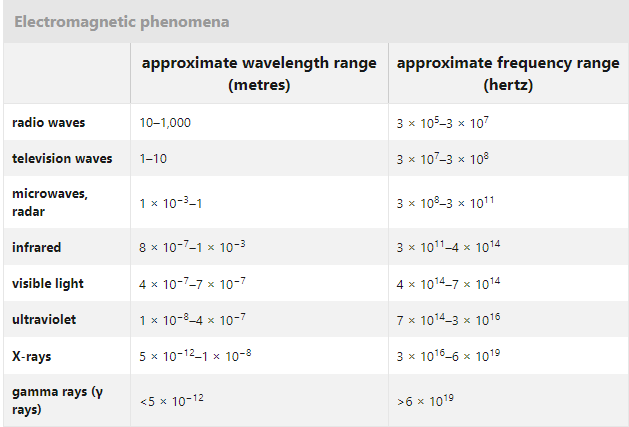
H2O has an angular structure | Why water has an angular structure?
A water molecule (H2O) can have a linear or angular structure, The dipole moments of the two O-H bonds in the structure being equal in magnitude and opposite in direction will cancel out. The net dipole moment (µ) would be zero.
In structure (b) the bond moments will add vectorially to give a definite net dipole moment. Since water actually has a dipole moment (1.85 D); its linear structure is ruled out. Thus water has an angular structure as shown in above Fig. . The dipole moments of the two O-H bonds in the structure being equal in magnitude and opposite in direction will cancel out. The net dipole moment (µ) would be zero.
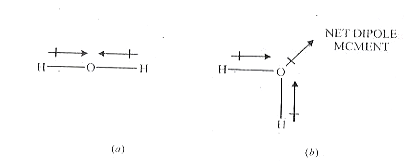
CO2 has a linear structure and SO2 has an angular structure.
Carbon dioxide has no dipole moment (µ = 0). This is possible only if the molecule has a linear structure and the bond moments of the two C=O units cancel each other.
On the other hand, SO2 has a dipole moment (µ = 1.63). Evidently, here the individual dipole moments of the two S=O bonds are not canceled. Thus the molecule has an angular structure. The vector addition of the bond molecule of the two S=O units gives the net dipole moment 1.63 D.
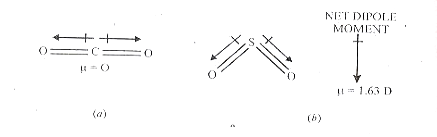
BF3 has a planar and NH3 has a pyramidal structure.
The dipole moment of the boron trifluoride molecule is zero. The three B-F bonds are arranged symmetrically around the boron atom in the same plane.
Identification of cis and trans isomers
The dipole moment can be used to distinguish between the cis and trans isomers. The cis isomer has a definite dipole moment, while the trans isomer has no dipole moment (µ =0) For example,
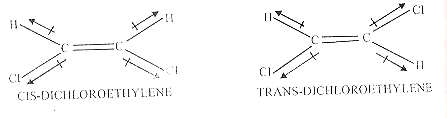
In the cis isomer, the bond moments add vectorially to give a net dipole moment. The trans isomer is symmetrical and the effects of opposite bond moments cancel so that µ = 0.
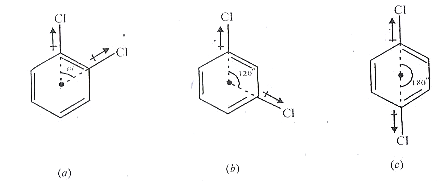
Identification of Ortho, Meta, and Para isomers in Benzene.
Benzene has dipole moment zero. Thus it is a planar regular hexagon. Let us examine the dipole moments of the three isomeric dichlorobenzenes (C6H4CI2). Since the benzene ring is flat, the angle between the bond moments of the two C- CI bonds is 60 for ortho, 120 for meta, and 180 for para. Fig.(a) (b), (c) respectively, On vector addition of the bond moments in each case, the calculated dipole moments are ortho 2.6 D, meta 1.5 D, and para 0 D.
CCl4 has zero dipole moment.
It can be explained on the basis of the symmetric tetrahedral structure of the molecule in spite of the C-Cl bond being highly polarized.
The percentage of ionic character can be calculated using dipole moment it is given by,
Ionic Character =
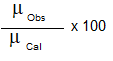
Polarization of Molecules :
When a molecule is placed in an electric field, the field distorts the electronic structure and changes the equilibrium positions of the nucleus giving rise to the separation of centers of positive and negative charges. The displacement of electronic charge is called electron polarization PE and that of nuclear charge is called atomic polarization PA. The sum of two values together is called distortion or induced polarization. PD is given by the equation.
PD = PE + PA
The electric field applied is responsible for induced polarization and the resulting dipole is called induced dipole moment u1, which is given by the equation.
µi = α E
Where alpha is a constant called the polarizability of the molecule and E is the electric field applied.

FAQs
What is molecular spectroscopy used for?
Spectroscopy is used in chemistry because all atoms and molecules have their own unique spectra. As a result, these spectra can be used to detect, identify and quantify the required information about the atoms and molecules.
What is an example of molecular spectroscopy? What are the types of molecular spectroscopy?
What is the principle of molecular absorption spectroscopy?
Atoms and molecules are excited from the ground state and undergo either resonant rotations, vibrations or electronic transitions, or excitations.
This web site is mostly a stroll-by for the entire information you wished about this and didn抰 know who to ask. Glimpse here, and you抣l positively uncover it.
Thank you dear
I抣l right away grab your rss feed as I can not find your e-mail subscription link or newsletter service. Do you’ve any? Please let me know so that I could subscribe. Thanks.
Thank you dear
wileetakkar@gmail.com
I and also my guys have already been viewing the excellent helpful hints found on your web page and then all of the sudden came up with a horrible feeling I never thanked you for those strategies. Most of the guys had been certainly excited to see them and have pretty much been tapping into these things. I appreciate you for getting considerably thoughtful as well as for going for these kinds of notable themes most people are really desperate to learn about. Our honest regret for not saying thanks to you sooner.
Thank you very much. Have a great day.
whoah this weblog is wonderful i like studying your articles.
Keep up the good work! You already know,
a lot of individuals are hunting round for this information, you can help them greatly.
Thank you very much
When some one searches for his necessary thing, therefore he/she
desires to be available that in detail, so that thing is maintained over here.
Very nice post. I just stumbled upon your blog and wanted to say that I’ve really enjoyed browsing your blog posts. In any case I抣l be subscribing to your feed and I’m hoping you write once more soon!
Hi, I think your website might be having browser compatibility issues. When I look at your website in Firefox, it looks fine but when opening in Internet Explorer, it has some overlapping. I just wanted to give you a quick heads up! Other then that, wonderful blog!
Thank you dear
There is noticeably a bundle to know about this. I assume you made certain nice points in features also.
thank you very much dear
I have recently started a website, the information you offer on this website has helped me greatly. Thank you for all of your time & work. “Yield not to evils, but attack all the more boldly.” by Virgil.
thank you very much dear
Pretty nice post. I just stumbled upon your weblog and wished to say that I’ve truly enjoyed surfing around your blog posts. After all I will be subscribing to your feed and I hope you write again soon!
thank you very much dear…
Just what I was looking for, thankyou for putting up.
thank you very much dear
Keep up the great piece of work, I read few articles on this site and I conceive that your site is real interesting and has got bands of great info .
Thank you