Hofmann Rearrangement | What Is Hofmann Rearrangement Reaction| Hofmann Reaction kya hai?
The conversion of amide to an amine with one carbon atom less by the action of alkaline hypohalite (NaOX or KOX) or bromine in alkali is known as Hofmann Rearrangement Reaction.
Hofmann Rearrangement Reactions
A rearrangement reaction is an organic reaction in which an atom, ion, group of atoms, or chemical unit migrates from one carbon atom to another carbon atom in the same or different molecule resulting in a structural isomer of the original molecule. The reaction often includes the breaking and/or making of carbon-carbon sigma bonds.
Types of rearrangement reactions.
Rearrangements are divided into two types,
a. Intramolecular rearrangement – Intramolecular rearrangements are those where the rearrangement of a group occurs within the molecule.
b. Intermolecular rearrangement – Intermolecular rearrangements are those where the rearrangement of a group occurs in the different molecules.
What is the Hofmann rearrangement reaction? What is Hofmann bromamide reaction? Illustrate Hofmann bromamide reaction ncert.
Principle of Hofmann rearrangement reaction
The conversion of amide to an amine with one carbon atom less by the action of alkaline hypohalite (NaOX or KOX) or bromine in alkali is known as Hofmann Rearrangement Reaction or Hofmann bromamide reaction.
This involves a rearrangement reaction of an N-haloamide into an isocyanate which hydrolyses rapidly, under the conditions of the reaction, into a primary amine. Because of the intermediate rearrangement, the reaction is also termed Hofmann haloamide rearrangement.
General Reaction

Mechanism – Hofmann rearrangement reaction
The reaction takes place in three steps.
Step I:- Formation of N- bromoamide
In the first step alkali (potassium hydroxide) reacts with halogen ( Bromine) forming hypohalite (KOBr = Potassium hypo bromide). This potassium hypo bromide reacts with amide forming N – bromoamide, this is also called Hofmann bromoamide reaction.
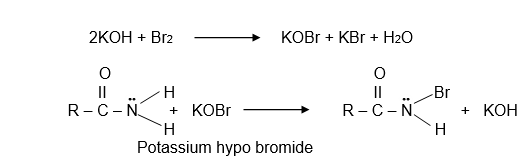
Step II:- Formation of isocyanate
In the second step, N- bromoamide reacts with alkali forming potassium halite which eliminates Br to give Nitrene, which is highly unstable. Hence rearrangement takes place with the migration of the ‘R’ (alkyl or aryl) group to the electron-deficient ‘N’ atom called Isocyanate.
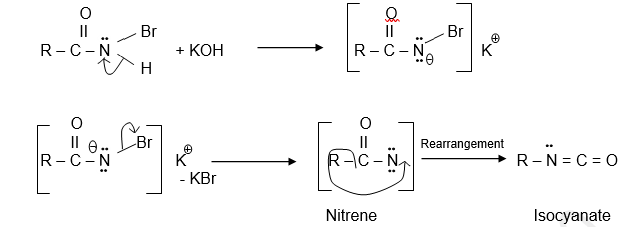
In aryl amides i.e., when the migrating group is aryl then the rate of Hofmann rearrangement gets increased by the presence of electron-releasing substituents in the para position of the aromatic ring.
In the cross-over experiment of Hofmann rearrangement, no cross-products are obtained when two different amides are rearranged then it is obvious that the rearrangement is intramolecular, and the migrating group never completely separates during the migration.
Step III:- Formation of Amine
In the third step, the isocyanate forms get reacted with an alkali-forming amine

The Curtius rearrangement is a similar well-known reaction.
Application of Hofmann Rearrangement Reaction
1) Preparation of aliphatic primary amine.
Hofmann Rearrangement is one of the most important rearrangement reactions used in the synthesis of amine. Hofmann Rearrangement is used in the preparation of aliphatic primary amine from amide. Large quantities of aliphatic amines are generally made synthetically using this method.

2) Preparation of aromatic primary amine
Hofmann Rearrangement is also used in the preparation of aromatic primary amine. Benzamide by using Hofmann rearrangement gives m-bromoaniline.
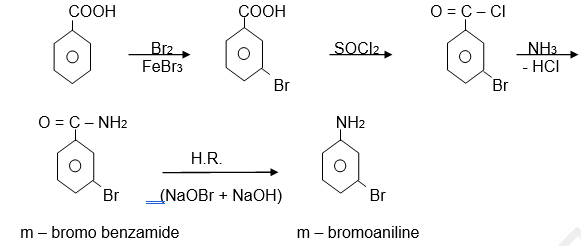
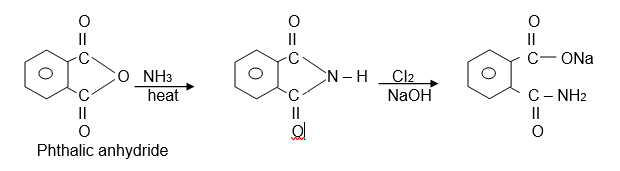
3) Preparation of anthranilic acid
Anthranilic can be prepared using the Hofmann Rearrangement reaction.

4) Preparation of heterocyclic amine–
Hofmann rearrangement reaction is used to prepare 3-amino pyridine from Nicotinamide, which is an important raw material in pharmaceutical industries and an Intermediate colorant. It is also used as a monomer in the polymer industry.
You can also read the Beckman Rearrangement reaction for a better understanding of the mechanism with electron-deficient N atoms.
Some important and Useful Articles
Mass spectroscopy is an instrumental method of structure determination. It gives information about the structure and molecular weight of the sample.
A molecule is bombarded with high energy electrons about 70 eV which leads to fragmentation. Mass spectra give exact molecular weight it also indicates hours the molecule breaks into fragments that can be recognized. Read More…
A polymer may be defined as a high molecular weight compound formed by the combination of small molecules of low molecular weight.
The small unit of which polymer is made is known as a monomer.
FAQs
Who discovered the Hofmann Bromamide reaction?
The Hofmann Bromamide reaction was discovered by famous German chemist August Wilhelm von Hofmann (8 April 1818 – 5 May 1892) and hence is named after him. He was awarded by Royal Medal in 1854 and the Copley Medal in 1875.
What is Hofmann Bromamide reaction with example ncert?
The conversion of amide to an amine with one carbon atom less by the action of alkaline hypohalite (NaOX) or bromine in alkali is known as Hofmann Rearrangement. In this N – bromoamide is formed which finally converts to amine hence this is also called the Hofmann bromoamide reaction. Examples are mentioned in the application.
What is Hofmann bromamide degradation reaction ncert ?
Hofmann developed the method for the preparation of primary amines. In this reaction, migration of an alkyl or aryl group takes place from the carbonyl carbon of the amide to the nitrogen atom. The amine so formed contains one carbon less hence the reaction is also called as Hofmann degradation reaction.
than that present in the amide
How is methylamine prepared from the Hofmann Bromamide reaction?
We can prepare Methylamine by Hofmann’s bromamide reaction using Acetamide.
CH3−CO−NH2 + Br2 + 4NaOH → CH3−NH2 + Na2CO3 + 2NaBr + 2H2O
What is meant by amide and amine?
Amide – An organic compound containing the group —CONH2, derived from ammonia by replacement of a hydrogen atom with an acyl group. General Formula as R-CONH2 . Example Benzamide ph-CONH2.
Amine – An organic compound containing the group —NH2, derived from ammonia by replacement of a hydrogen atom with an alkyl or aryl group. General Formula as R-NH2. Example Ethylamine (C2H5NH2).
What are Nitrenes in chemistry?
Nitrenes are sextet, highly reactive molecular species with the monovalent nitrogen atom, which can exist in a singlet or a triplet state.
What Is Hofmann Rearrangement Reaction?
The conversion of amide to an amine with one carbon atom less by the action of alkaline hypohalite (NaOX or KOX) or bromine in alkali is known as Hofmann Rearrangement Reaction.

Keep working ,terrific job!
thank you dear
Wow! This could be one particular of the most useful blogs We’ve ever arrive across on this subject. Actually Great. I am also a specialist in this topic therefore I can understand your hard work.
Your home is valueble for me. Thanks!?
I’m often to blogging and i really admire your content. The article has really peaks my interest. I’m going to bookmark your web site and maintain checking for new information.
Useful info. Fortunate me I found your website by chance, and I’m surprised why this accident didn’t came about in advance! I bookmarked it.
I was curious if you ever considered changing the layout of your blog? Its very well written; I love what youve got to say. But maybe you could a little more in the way of content so people could connect with it better. Youve got an awful lot of text for only having one or two images. Maybe you could space it out better?
Magnificent beat ! I wish to apprentice even as you amend your site, how can i subscribe for a blog website? The account helped me a acceptable deal. I had been a little bit acquainted of this your broadcast offered vibrant clear idea
My developer is trying to convince me to move to .net from PHP. I have always disliked the idea because of the costs. But he’s tryiong none the less. I’ve been using Movable-type on a variety of websites for about a year and am worried about switching to another platform. I have heard fantastic things about blogengine.net. Is there a way I can transfer all my wordpress posts into it? Any help would be greatly appreciated!
I like what you guys are up too. This kind of clever work and exposure! Keep up the very good works guys I’ve you guys to our blogroll.
Very great post. I just stumbled upon your weblog and wished to say that I have truly enjoyed surfing around your blog posts. After all I will be subscribing to your rss feed and I am hoping you write once more soon!
There are some attention-grabbing deadlines in this article however I don抰 know if I see all of them heart to heart. There may be some validity but I will take maintain opinion until I look into it further. Good article , thanks and we wish more! Added to FeedBurner as nicely
It抯 actually a cool and useful piece of information. I抦 glad that you shared this useful information with us. Please keep us up to date like this. Thanks for sharing.
I抳e been exploring for a little bit for any high-quality articles or blog posts in this kind of space . Exploring in Yahoo I eventually stumbled upon this website. Reading this info So i am happy to show that I’ve an incredibly just right uncanny feeling I came upon exactly what I needed. I most without a doubt will make certain to do not disregard this site and provides it a glance on a continuing basis.
I am often to running a blog and i really appreciate your content. The article has really peaks my interest. I am going to bookmark your site and preserve checking for brand new information.
Howdy! Someone in my Facebook group shared this site with us so I came to take a look. I’m definitely enjoying the information. I’m book-marking and will be tweeting this to my followers! Superb blog and outstanding style and design.
Thank you very much dear
Thank you, I’ve just been searching for information about this topic for ages and yours is the greatest I have discovered so far. But, what about the conclusion? Are you sure about the source?
Yes..Thank you for your comment
I precisely needed to appreciate you yet again. I’m not certain the things that I could possibly have carried out in the absence of the entire points shared by you on this area of interest. It was the intimidating circumstance for me, however , finding out the very expert manner you handled that made me to jump over joy. I am grateful for this assistance and in addition wish you comprehend what a powerful job you are always providing instructing others all through your blog post. I’m certain you haven’t come across all of us.
Excellent post. I used to be checking constantly this blog and I’m inspired! Very useful info particularly the remaining part 🙂 I maintain such info a lot. I was seeking this particular info for a very long time. Thanks and good luck.
Superb post however I was wanting to know if you could write a litte more on this subject? I’d be very grateful if you could elaborate a little bit more. Many thanks!
Cool blog! Is your theme custom made or did you download it from somewhere? A design like yours with a few simple tweeks would really make my blog stand out. Please let me know where you got your theme. Thank you
You really make it seem so easy with your presentation but I find this matter to be really something which I think I would never understand. It seems too complex and very broad for me. I’m looking forward for your next post, I will try to get the hang of it!
I like what you guys tend to be up too. Such clever work
and reporting! Keep up the awesome works guys I’ve added you guys to my
personal blogroll.
Have you ever considered about adding a little bit more than just your articles? I mean, what you say is important and everything. But think about if you added some great photos or video clips to give your posts more, “pop”! Your content is excellent but with pics and videos, this website could definitely be one of the most beneficial in its field. Good blog!
Thank you dear
Great beat ! I would like to apprentice while you amend your site, how can i subscribe for a weblog website? The account helped me a appropriate deal. I were a little bit familiar of this your broadcast offered vibrant clear concept
Thank you dear
Hi, just required you to know I he added your site to my Google bookmarks due to your layout. But seriously, I believe your internet site has 1 in the freshest theme I??ve came across. It extremely helps make reading your blog significantly easier.
I have been checking out a few of your articles and i must say pretty nice stuff. I will definitely bookmark your site.
Thank you
I view something genuinely interesting about your weblog so I saved to bookmarks.
Thank you dear
Keep up the fantastic work, I read few blog posts on this internet site and I believe that your blog is very interesting and has got circles of excellent information.
Thank you dear
I wanted to thank you for this great read!! I definitely enjoying every little bit of it I have you bookmarked to check out new stuff you post…
Thank you dear
I am really enjoying the theme/design of your site. Do you ever run into any internet browser compatibility issues?
A number of my blog visitors have complained about
my site not operating correctly in Explorer but looks great in Safari.
Do you have any suggestions to help fix this issue?
Thank you very much dear
Im happy I found this blog, I couldnt learn any information on this subject matter prior to. I also run a site and if you want to ever serious in a little bit of guest writing for me if feasible really feel free to let me know, im always appear for people to test out my site. Please stop by and leave a comment sometime!
Thank you sir
Awsome blog! I am loving it!! Will be back later to read some more. I am taking your feeds also.
Thanks