ATOMIC ABSORPTION SPECTROSCOPY (A.A.S) | APPLICATION, INSTRUMENTATION & ADVANTAGES OF ATOMIC ABSORPTION SPECTROSCOPY
Principle of atomic absorption spectroscopy (AAS)
There are two main types of Atomic spectroscopy namely Atomic emission spectroscopy- Flame emission spectroscopy (Flame photometry) and Atomic absorption spectroscopy (AAS).
Atomic Absorption Spectroscopy is the study of the absorption of radiation by the atom in the ground state when a sample is introduced into flame.

When an element is introduced into the flame, a few of its atoms absorb thermal energy from the flame and get excited but the majority of its remains in the ground state. The atom which remains in the ground state is capable of absorbing its characteristic radiation. It is the study of the absorption of radiation by the atom in the ground state when a sample is introduced into flame.
This absorption of characteristic radiation forms the basis of qualitative analysis of elements under study since every element absorbs its own characteristic radiation. The difference in intensity of incident radiation and emitted radiation gives the intensity of absorption which in turn gives the number of atoms in the sample at the ground state and hence forms the basis of quantitative analysis. Hence AAS is an invaluable tool for quantitative and qualitative analysis of a large number of elements. This elegant method was discovered by Sir. Allan Walsh in 1955.
Example: When a beam of radiation is passed through a flame containing the atoms of an element in the ground state, the atom will absorb their characteristic wavelength for e.g. Sodium will absorb the wavelength radiation of 589 nm and 589.6 nm.
If the beam emerging from flame is analyzed in the spectrometer, an absorption spectrum consisting of dark lines against a bright background is observed. The dark line corresponds to the absorption of characteristic radiation by an atom of the element. The sodium absorption spectrum consists of two dark lines corresponding to 589 nm and 589.6 nm against a bright background. The dark line is due to the absorption of light used for electronic transition.
Atomic absorption spectroscopy instrumentation
Source of radiation–
In AAS, the radiation source is very important. The selected source of radiation should emit the radiation which can excite the atom of the element from the state to the excited state i.e. they should give resonating radiation.
Hallow cathode Lamp in Atomic absorption spectroscopy
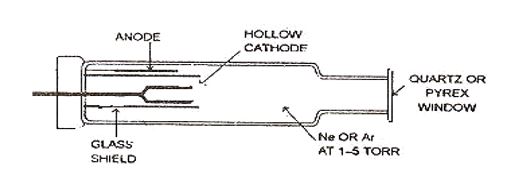
A Hallow cathode Lamp is a type of cold cathode lamp used as an ideal source of radiation. It consists of a hollow cathode cup that is made up of the element under study (e.g. if the copper sample is under study then the cathode cup is made up of copper and hence will give a resonating radiation for Cu.)
The cathode cup (made up of under study) is protected in a glass shield. The tungsten wire is made to act as the anode. Both electrodes are enclosed in a glass envelope containing an inert gas like neon or argon at low pressure. An electrical field of very high potential is applied across the electrodes, because of which the atom of inert gas gets ionized, and positively charged ions are attracted toward the cathode. These fast-moving ions collide with the cathode surface and remove the metal atom from the surface. This process is called Sputtering.
This removal of the metal atom now collides with a highly energetic gas ion that gets excited, these excited atoms radiate their characteristic radiation while coming back to the ground state because of which a glow appears inside cathode cups. Cathode cups which are made up of alloy can be used for multi-elemental analysis.
The neon or argon gas filled in the hollow cathode lamp performs three functions,
It is the main source of current carrying capacity in the hollow cathode.
It dislodges (removes) atoms from the surface of the cathode.
It is primarily maintained in the hollow cathode lamp is 1 to 5 torr.
Each hollow cathode lamp emits the spectrum of the metal which is used in the cathode. For example, a copper cathode emits the copper spectrum; a Zinc electrode emits the zinc spectrum, and so on. For this reason, a different hollow cathode lamp has to be used for each element to be analyzed by atomic absorption spectroscopy.
Rotating chopper
Resonating radiation is emitted by the hallow cathode lamp as well as by the atoms present in the flame (i.e. due to thermal excitation as in FEP) and the photo tube responds to radiation from the flame as well as from hollow cathode lamp and hence an interference is created in absorption measurement. This problem is solved by using a device called the Rotating chopper.
A chopper is a rotating mirror of such a shape that it permits the beam to be reflected back. This produces an intermittent pulsating beam of light. Such a beam produces an alternating current in the photomultiplier tube while the flame will produce a direct current in the photomultiplier tube. The direct current is not amplified since the amplifier is tuned to amplify only the alternating current coming from the Hallow cathode lamp.
Burner
The burner is used for the atomization of the sample two types of atomizers can be used, flame & non-flame. In the flame type of atomizer, a flame is produced by burning a fuel-oxidant mixture. The sample is a nebulizer in flame where the atomization of the sample takes place. The gaseous atom in the ground state absorbs the characteristic radiation coming from the hollow cathode lamp. Flame Burners used are Total consumption burners and Pre-mix burners.
The sensitivity of flame atomizers (burner) is less due to some of their limitation and the fraction of sample which is atomization stays in the path of the optical beam by flame for fraction of a second. The non-flame atomizer used is called an Electro thermal atomizer.
Monochromator
The filter allows the radiation characteristic of the element to pass through and will absorb all other unwanted radiations. If the reflected beam consists of a large number of wavelengths, a monochromator (Grating or Prism) is used to disperse the reflected beam into its individual wavelengths. When a monochromator is rotated, different wavelengths are focused one by one on the photocell. This scanning enables us to detect several elements simultaneously.
Photocell or Photomultiplier Tube
The beam from the monochromator falls on the photocathode of the photocell. The resultant electric current is amplified and its magnitude is read. For more sensitive measurements a photomultiplier tube is used.
Detector
The electric current amplified by a photo multiplayer tube is measured and its magnitude is read out on the meter. The magnitude of the electric current produced is directly proportional to the concentration element.
Electrothermal atomizer
The electrothermal atomizer, also called graphite furnace or non-flame atomizer consists of a graphite tube 5cm long, 1cm in internal diameter & open at both ends, Tube has a central hole for a micropipette. Two electrodes are fitted at the end of the tubes. Radiation from the source passes along the axis of the tube. A stream if inert gas like argon or nitrogen flows in and around the tube to prevent possible oxidation of graphite metal. The entire assembly is kept in a water-cooled metal jacket.
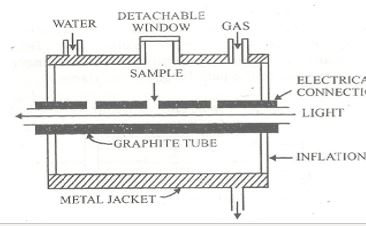
A few microliters sample is introduced through the central window into a graphite tube & electrically heated in the least three stages.
Drying stage
The sample is first heated at a temperature slightly above the boiling point of the solvent to remove the solvent by evaporation.
Ashing stage
The temperature is raised to a value that is sufficient to remove all volatile components of the sample but not sufficient to vaporize the analyte. Typically the temperature in the ashing stage is maintained between 600K to 1500K for 45 secs.
Atomization stage
The temperature is increased to a point at which discrete (gaseous) atoms of analyte are formed. Typically the temperature in this stage is maintained between 2300K to 3300K for a period of 5s. The absorption of the analyte is measured during the atomization stage. Electrothermal atomizers provide enhanced sensitivity because the entire sample is atomized in a short period & average residence time of the atom in the optical path is a second or more.
Advantages of Atomic Absorption Spectroscopy A.A.S.
The AAS technique is very specific as the atom of a particular element absorbs its own characteristic wavelength. Hence spectral interference is less than in flame photometry.
A large number of ground-state atoms absorb radiation. Thus, temperature changes do not affect absorption. In flame photometry, a small number of excited atoms emit light depending on the flame temperature.
AAS is more sensitive for Ag, B, Bi, Cd, and Fe. Flame photometry is more sensitive for Al, Ba, Ca, K, and La. Both techniques are equally sensitive to Cu, Cr, Mn, Mo, and N.
Application of Atomic Absorption Spectroscopy A.A.S
Identification of elements – The use of AAS in this field is limited as for the analysis of each element a separate hollow cathode lamp is needed. However, with the introduction of multi-element lamps. It is possible to detect different present in a sample.
AAS is used to estimate trace metal in clinical and biological substances, to estimate Na and K in blood serum and to estimate lead in petrol.
AAS is used to detect Mo, W, and V in steel samples and a trace of elements in forensic materials, soils, fertilizers, and industrial effluent.
AAS is used to detect toxic metals like Cu, Ni, and Zn in a food product. Hg which toxic metal can also be determined in traces in a food sample and in cases of mercury poison.
AAS is an invaluable tool in the study of pollution and adulteration of food articles and drugs.
AAS can be used in Quantitative Analysis.
FAQs
Atomic absorption spectroscopy principle?
Atomic Absorption Spectroscopy is the study of the absorption of radiation by the atom in the ground state when a sample is introduced into flame.
What is the difference between AAS and atomic emission spectroscopy?
In flame emission spectroscopy, certain wavelengths of light emitted from atoms are recorded, whereas, during AAS, certain wavelengths of light absorbed are recorded.
What is the Principle of spectroscopy?
Spectroscopy is the study of the interaction between matter and radiation.
One thought on “ATOMIC ABSORPTION SPECTROSCOPY (A.A.S) | APPLICATION, INSTRUMENTATION & ADVANTAGES OF ATOMIC ABSORPTION SPECTROSCOPY”