7 Application of mass spectroscopy – spectrometer uses, instrumentation of mass spectroscopy with the basic principle
Before learning the application of mass spectroscopy, let’s know the basics.
Mass Spectroscopy Principle
Mass spectroscopy is an instrumental method of structure determination. It gives information about the structure and molecular weight of the sample.
A molecule is bombarded with high energy electrons about 70 eV which leads to fragmentation. Mass spectra give exact molecular weight it also indicates hours the molecule breaks into fragments that can be recognized.

Sub. e– beam cation

Fragmentation of M+ gives many cations and radicals. Cations are accelerated, deflected (by electric and magnetic fields) detected, and recorded. A mass spectrum is a plot of the m/z value of various fragment cations against their relative abundances the peak obtained due to molecular ions is called the molecular ion peak. Its m/z value, z usually gives us the molecular weight of the substance.
It is usually the last peak (the last cluster of peaks). It becomes a base peak it is very stable the fragment forming the base peak is very stable. The most abundant peak is called the base peak. It is arbitrarily assigned 100% and relative abundances of other fragments are recorded w.r.t. it.
Basic Principle and Theory of Mass Spectroscopy
1. The mass spectrometer is an instrument that helps in separating individual atoms or molecules because of the difference in their masses.
2. Consider molecule M, which is bombarded with a beam of electrons.
3. Suppose this is ionized as follows,

4. Where M+ is an ionized molecule and e– is an electron. The ions are then accelerated in an electric field at voltage V.
5. If this is the condition, the energy given to each particle is eV and this is equal to the kinetic energy which is equal to 1/2 mV2
6. This can be explained as,
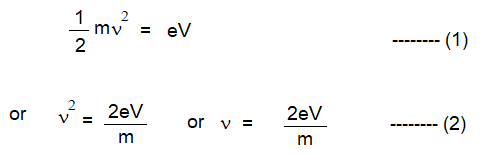
7. Where is the velocity of the particle of mass m, e is the charge on an electron and V is the accelerating voltage.
8. All the particles possess the same energy eV.
9. Also, all particles have the same kinetic energy of 1/2 mV2
10. As the value of m varies from particle to particle, the velocity v also changes such that remains 1/2 mV2 constant.
11. For a particle of mass m1 and velocity v1 equation (1) becomes as,

Similarly for a particle of mass m2 and velocity v 2 equation (1) becomes as,

For particles m3 , m4 ……. Of velocities V3, V 4respectively, equation (1) becomes as,

From equations (3), (4), (5), (6), and (7), we have,

12. From equation (8), it follows that the velocity of different particles will vary, depending on the mass of the particles.
13. The charged particles accelerated by an applied voltage enter a magnetic field H.
14. This field attracts the particles and they move in a circle around it. This attractive force, due to the magnet is HeV, whereas the balancing centrifugal force of the particles is mv2 /r
When the particles start moving uniformly around the circular path, the two forces become equal, i.e.
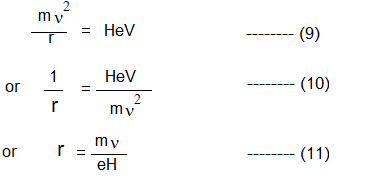
Where r is the radius of the circular path of the particle.
On substitution equation (10) in (2), we get,
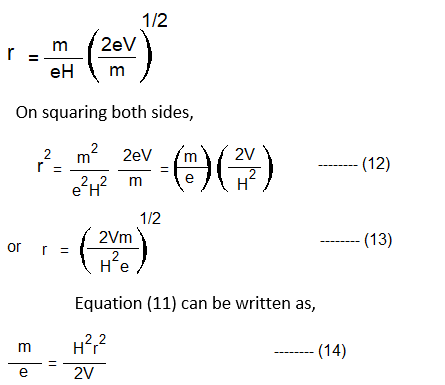
16. From equation (12), it follows that the radius of the circular path of particles depends on the accelerating voltage V, the magnetic field H, and the ratio,m\e
17. As e, V, and H are constant, it means that the radius of the ionized molecule depends on m, its mass.
18. The relation between m, the radius of the circular path of the ion, V, and H, i.e. equation (12), is the basis of the separation of particles according to their masses.
19. Thus, the radius of the ion path may be changed by varying either the magnetic field (H) or the accelerating voltage (V).
20. By either method, ions of different mass-to-charge ratios (m/e) can be made to impinge upon the collector, in turn thus, giving rise to a spectrum (fig).
21. Magnetic field variation enables a wide range to be covered in a single sweep, but for very rapid scanning a voltage sweep must be employed.
Instrumentation of Mass spectrometer
The mass spectrometers used for the investigation of any compound may vary in their types but generally, all contain the following components.
a) The inlet system (or sample handling system)
b) The ion source (or ionization chamber)
c) The electrostatic accelerating system.
d) The ion separator.
e) The ion collector (The detector and read-out system)
f) The vacuum system.
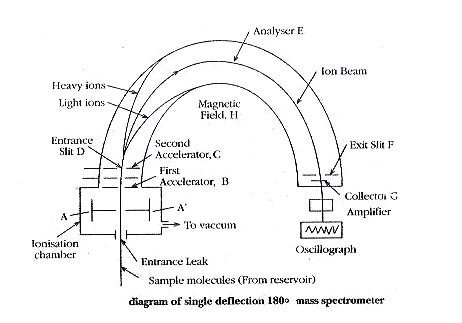
The ionization chamber produces a beam of positive ions of different e/m values. The beam is forced to pass through the slit of plates A and A’ having low potential. This beam is then accelerated in the electrical field between B and C. The ions emerge out through D to pass through the evacuated tube E which is kept in strong magnetic field H, perpendicular to the plane of the diagram.
Only those ions having an e/m ratio of 2E / H2r2 will be allowed to pass through the exit slit I and strike collector plate G. In order to bring ions with different values of e/m to the same focus, where the collector plate is placed, the value of E is changed keeping the value of H constant. Thus, the particles with different e/m values are made to follow the same path in the mass spectrometer.
Components of a mass spectrometer
Inlet system (or sample handling system) :
The sample is first converted into vapour. For mass spectrometry, a sample size of about 1 µ mole is introduced. Only a few percent of this will actually enter the ionization chamber and about 0.1% is ionized in the ionization chamber.
The ion source (or Ionisation chamber) :
The positive ions, produced by either heating salts of metallic elements or bombarding them with low energy electrons, are forced through the slit of the first accelerating plates A and A’ by applying a small potential difference between the plates.
The Electrostatic Accelerating System :
In this, the ions attain their high velocities by applying a high potential between the first accelerator plate B and accelerator plate C. Thus, the energy of the ions will be increased resulting in well-defined fragments or molecules. Each fragment carries usually a unit positive charge. In most spectrometers, the potential between the accelerator plates provides a means whereby particles of a particular mass are focused on the plate.
Ion separator (Analyser) :
In this region, ions are separated according to their masses. An analyzer must have a high-resolution power and a high rate of transmission of ions.
Ion collector :
The ion beam currents are of the order of 10-15to 10-20ampere. An electron multiplier tube is attached to amplify the current. The read-out display usually possesses a direct recording oscillograph where ion current or relative intensity is plotted against e/m.
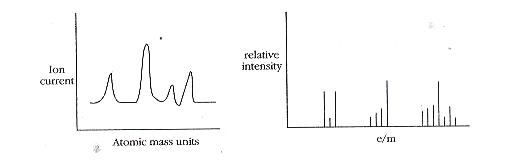
In this, the number of peaks will correspond to the number of fragments present. From the value of the field strength at which the peak occurs, the mass numbers are evaluated. The ratio of peak height to field strength gives a relative abundance of isotopes.
The vacuum system :
A high vacuum is to be maintained in the instrument. The oil diffusion and mercury diffusion pumps are commonly used in different combinations to decrease the pressure as low as possible.
Applications of Mass Spectrometry – Spectrometer uses
The following are the applications of mass spectroscopy (Spectrometer uses)
1. Molecular Weight Determination :
The molecular weight of compounds can be determined easily. This method gives the molecular weight of the compound to the nearest whole number.
2. Determination of Molecular Formula
The molecular formula of the compound can be determined by identifying the molecular ion peak and its position in the spectrum accurately.
3. Identification of compounds from fragmentation patterns :
In mass spectrometers, the fragmentation of even simple molecules produces a large number of ions with different masses. A complex structure is obtained for a molecule which is very useful for the identification of a compound or functional group.
4. Isotopic abundance measurement :
In mass spectra of ion current against atomic mass units, the ratio of peak height to field strength is given the relative abundance of isotopes. These isotopes are used in the determination of the formula of organic compounds, isotopic dilution technique, tracer technique, and determination of the age of rocks and minerals.
5. Identification of cis and trans Isomers :
The isomeric compounds give similar mass spectra. The molecular ion peak for the trans isomer is found to be more intense than that for cis isomers. This distinction successfully allows the identification of cis and trans isomers.
6. Impurity Detection :
This method is used to detect impurities up to part per million level.
7. Characterization of polymers :
In this, the polymer is first decomposed by heating. The products formed are studied in the mass spectrometer. The spectrum gives characteristic information concerning the structure of the polymer. e.g. mass spectrometry gives information on chlorine whether occurs randomly or in blocks in the structure of chlorinated polymers.
Nowadays, mass spectrometry is also used in the determination of ionization potential, bond dissociation energy, and latent heat of vaporization of liquids.
Useful Content
A polymer may be defined as a high molecular weight compound formed by the combination of small molecules of low molecular weight.
The small unit of which polymer is made is known as a monomer.
The reaction by which monomers combine to form a polymer is known as polymerization. Read More..
Photochemistry is the study of chemical reactions caused by the absorption of light.
The most familiar photochemical reactions in photochemistry are….
FAQs
What are the 4 main stages in mass spectrometry?
Mass spectrometry is divided mainly into four stages 1. Ionization, 2. acceleration of charged particles, 3. deflection in an electric and magnetic field, and 4. Analysis at the detector.
What is the application of mass spectroscopy?
There is various application of mass spectroscopy. Please refer above article for more details.
What is the basic principle of mass spectrometry?
Mass spectroscopy is an instrumental method of structure determination. It gives information about the structure and molecular weight of the sample.
Who invented the mass spectrometer?
Sir J.J. Thomson, in 1912.
What is NMR spectroscopy? Can mass spectroscopy be used in organic chemistry?
NMR Spectroscopy is one of the most important and powerful techniques used in organic chemistry for the structural elucidation of Organic (and inorganic) compounds and biomacromolecules. Mass spectroscopy also can be used in organic chemistry.

One thought on “7 Application of mass spectroscopy – spectrometer uses, instrumentation of mass spectroscopy with the basic principle”