Photochemistry | Principles, and applications of photochemistry | photochemistry and photophysics concepts research applications
Photochemistry is the study of chemical reactions caused by the absorption of light.
The most familiar photochemical reactions in photochemistry are:-
a. Photosynthesis: –
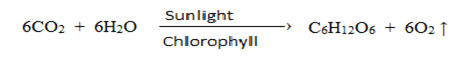
b. Hill reaction: ( Photolysis of water )
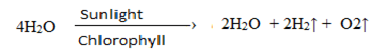
Principle of Photochemistry
A molecule absorbs a photon of light gets excited due to absorbed light energy and then undergoes a chemical reaction. It is applicable to individual molecules. The excitation is highly selective. The law of photochemical equivalence states that one molecule would react for each quantum absorbed.
The efficiency of a photochemical reaction is expressed as quantum yield.

Photosensitization
In this process, one molecule (known as a doner) absorbs light energy gets excited and transfers its energy to another molecule (this molecule is called an acceptor). Thus the donor molecule returns to the ground state while the accepter gets excited which undergoes a photochemical reaction. This transfer of energy from donor to accepter followed by emission of light or reaction of accepter is called photosensitization.
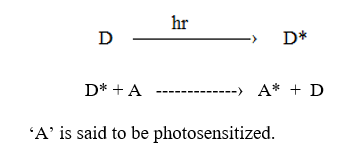
Molecules such as benzophenone, acetophenone, and mercury are used as photosensitizers e.g. conversion of 1, 5 hexadiene to bicyclic compound.
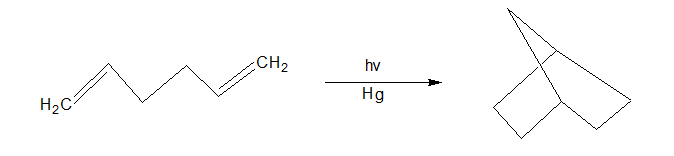
Selection Rule
All types of electronic transition may not be possible. During electronic excitation, their spin multiplicity remains the same.
The transitions between the states or the same multiplicity are permitted transitions.
Singlet ——-› Singlet(S0 ——-› S1) or
Triplet ——-› triplet are permitted transition.
The transitions between states to triplet are less probable and one is called forbidden transitions.
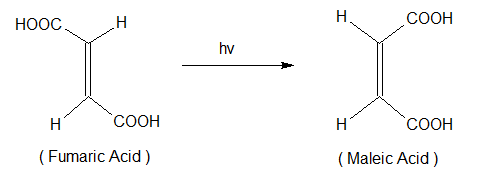
Photochemical Isomerisation
In photochemistry, photochemical reactions are common in photochemistry and involve interconversion of geometrical isomerization of olefinic compounds. These reactions are promoted by direct irradiation of the substrate or by photosensitized energy transfer. The ‘e’ isomer can be converted into a thermodynamically less stable “z” isomer by direct irradiation.
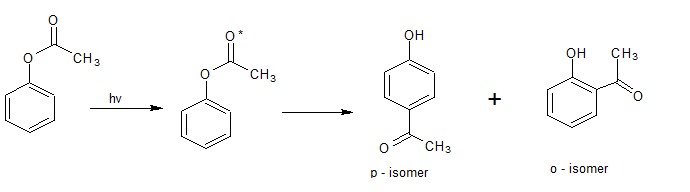
Molecular rearrangement can also be promoted photochemically phenolic esters rearrange on irradiation giving ‘O’ and ‘P’ acyl phenols. This rearrangement is known as photo tries rearrangement and reaction taken by the photoinduced free radical process. e.g.
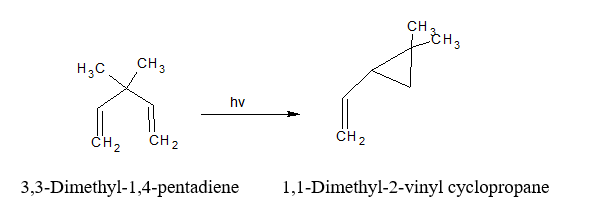
Photochemical rearrangement of 1,4- dienes:- (di-pi-methane rearrangement in photochemistry)
The di-pi-methane rearrangement is a photochemical reaction that contains two pi double bond systems which are separated by a saturated carbon atom (a 1,4-diene or an alkyl-substituted aromatic ring), to form an alkene (or aryl-) substituted cyclopropane.
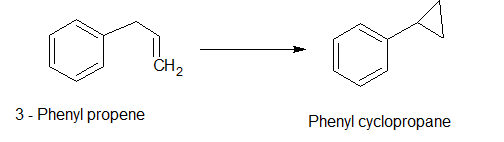
Another example is the rearrangement of allyl benzene to phenyl cyclopropane.
Jablonski Diagram
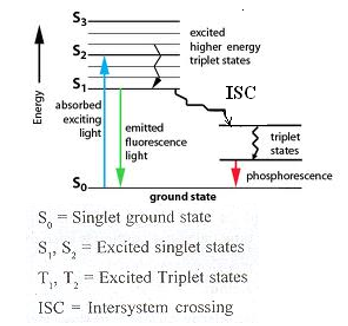
In photochemistry, the various processes taking place after a molecule absorbs light energy are summarized as follows.
Electronic Excitation from S0 to S1or S1 to S2
Such a type of excitation is observed when molecules absorb light energy. When there is higher absorption of light the electron may excite to S2 level also.
Vibrational Relaxation
The electronic levels are subdivided into small energy levels which are called vibrational energy levels. The electrons also possess this type of energy. But when an electron from a higher vibrational level loses its energy, it comes to a lower vibrational level. ( Eg V2 to V1)
Internal Conversion from S2 to S1 to S0
When molecules further lose energy and come to the ground stage such a type of conversion is observed.
Fluorescence S1 to S0
The molecule releases the energy from S1 and comes to the S0 stage which is the ground stage. This process occurs with visible radiation which is called Fluorescence. Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. This phenomenon continues till light is present and stopped as the light is cut off.
Intersystem crossing ( ISC) S1 to T1 transition
When a singlet state nonradiative transitions to a triplet state, or conversely triplet transitions to a singlet, that process is known as an intersystem crossing. The probability of this process
occurring is more favorable when the vibrational levels of the two excited states overlap since little or no energy must be gained or lost in the transition. As the spin/orbital interactions in such molecules are substantial and a change in spin is thus more favorable, the intersystem crossing is most common in heavy-atom molecules.
Phosphorescence
Phosphorescence is a specific type of photoluminescence. In Phosphorescence, the substance does not re-emit the radiated light absorbed. The slower time scales of the re-emission are associated with forbidden energy state transitions in quantum mechanics. It is a delayed process and this transition occurs very slowly.
Singlet and triplet states
Most organic compounds have an even no of electrons and are paired with opposite spins. The net spin is zero such an electronic state is called a singlet state. Most of the organic molecules in the ground state are in the singlet state. When a molecule absorbs light it is excited to a singlet state (S7) of the molecule. This state is short-lived and has the molecule returns to the ground state by emission of a photon or it may transfer energy to another molecule or it may undergo a chemical reaction such as decomposition or rearrangement.
If ‘S’ is the total (net) spin the spin multiplicity is 2S + 1
For the singlet state spin multiplicity is zero.
If electrons have parallel spins they are unpaired. The electronic spins do not cancel each other. E.g. for two unpaired electrons.
Net spin (S):(+ ½ + ½) =1
Spin multiplicity = 2S + 1 =2 x 1 + 1 = 3
This magnetic state is called triplet state.
Photoreduction
In photochemistry, photoreduction of the ketone is an important example of a photochemical solution of benzophenone that can be reduced by exposing a solution of benzophenone and benzhydrol in benzene to ordinary sunlight for a few days. It takes place as follows.
Benzophenone absorbs light and undergoes n → π* transition which then undergoes intersystem crossing to a triplet state.
Photoreduction example
We can easily understand it by referring to the following Photoreduction example,
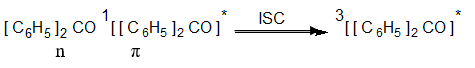
It then abstracts hydrogen from benzhydrol to give two diphenyl hydroxyl methyl radicals.
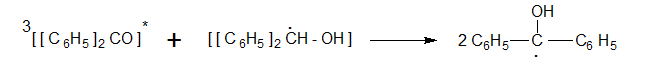
These two radicals combine to give Benz pinacol
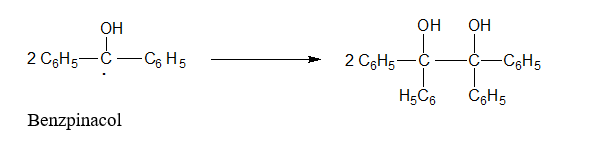
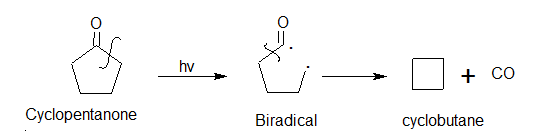
Norrish Type-1 reaction
The tendency of ketone to undergo hemolytic cleavage of the α– carbon cleavage bond is called Norrish type 1 or α– cleavage.
As the bond dissociation energy of a C-C bond adjacent to a carbonyl (-C=0 ) has been comparatively small, photochemical excitation of ketones usually causes the hemolytic fission of the αcarbon – carbon bond.
The most studied example has been acetone which gets photolyzed in the vapour phase as well as in the liquid phase. Absorption of light undergoes a C – C cleavage to form variable products. The products formed depend on temperature.
At higher temperatures ( 1000 C)
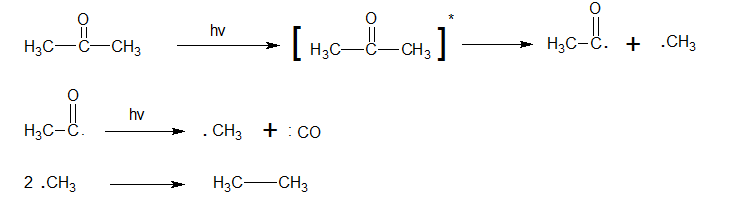
At temp. above 100oC acetyl radicals get decarboxylated with the ultimate formation of ethane and carbon monoxide.
At room temperature
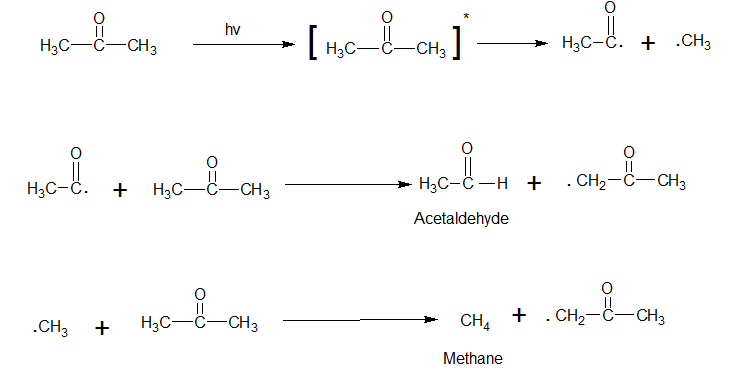
Cyclic Ketones undergo α- cleavage to form a biradical which eliminates CO and then cyclizes into cycloalkanes.
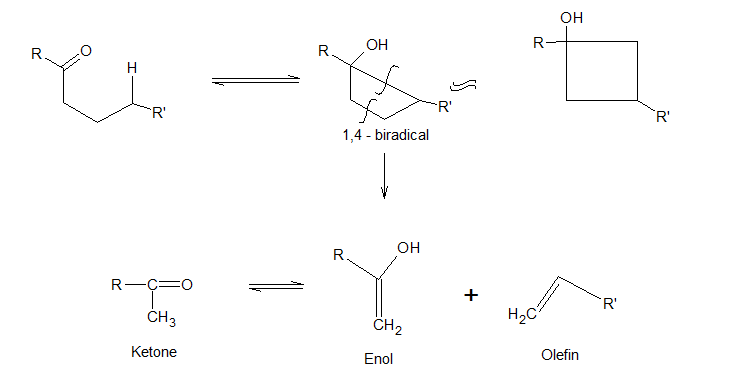
Norrish Type 2 reaction
Ketones having ‘γ hydrogen atom’ undergo Norrish type 2 reaction to give an olefin and enol of the smaller ketone. Spectroscopic studies have demonstrated that the disappearance of the starting ketones takes place at the same rate as the appearance of enol form. The reaction takes place by the initial ‘hydrogen abstraction’ by the oxygen atom forming a 1, 4 – biradical which subsequently cleaves to yield olefin and enol.
Useful article – TWELVE PRINCIPLES OF GREEN CHEMISTRY
FAQs
What is Grotthuss–Draper laws of photochemistry?
Grothus-Draper provided the law at the beginning of the 19th century which states that “When light falls on any substance or materials, not all but only a fraction of it is absorbed whereas the rest is either reflected or transmitted, only that light which is absorbed by a system can bring about a photochemical reaction. This law is also called the Grotthuss–Draper law.
What is Stark-Einstein Law of photochemistry?
Stark-Einstein Law is the second law of photochemistry. According to this law, one molecule is activated by the absorption of one quantum of radiation in the first step of a photochemical reaction.
What is photochemistry?
Photochemistry is the study of chemical reactions caused by the absorption of light.
I like this web site so much, saved to favorites. “Respect for the fragility and importance of an individual life is still the mark of an educated man.” by Norman Cousins.
Thank you very much, dear..
I think you have mentioned some very interesting details, thanks for the post.
Thank you very much, dear..
Cool blog! Is your theme custom made or did you download it from somewhere? A theme like yours with a few simple tweeks would really make my blog jump out. Please let me know where you got your theme. Thank you
Thank you very much, dear..
Fantastic site. Lots of useful information here. I am sending it to some pals ans also sharing in delicious. And naturally, thanks for your sweat!
Thank you very much…
Wonderful web site. A lot of helpful information here. I¦m sending it to a few buddies ans also sharing in delicious. And certainly, thanks on your sweat!
Thank you dear
I have been exploring for a little for any high quality articles or blog posts on this kind of area . Exploring in Yahoo I at last stumbled upon this site. Reading this information So i am happy to convey that I have an incredibly good uncanny feeling I discovered just what I needed. I most certainly will make certain to don’t forget this web site and give it a glance on a constant basis.
Thank you dear
Good ?V I should certainly pronounce, impressed with your website. I had no trouble navigating through all tabs as well as related information ended up being truly easy to do to access. I recently found what I hoped for before you know it in the least. Quite unusual. Is likely to appreciate it for those who add forums or anything, site theme . a tones way for your client to communicate. Excellent task..
Thank you dear
Thank you for every other excellent post. Where else could anybody get that kind of info in such an ideal means of writing? I have a presentation next week, and I’m at the look for such info.
Thank you dear
Thank you for sharing with us, I think this website really stands out : D.
There are some fascinating cut-off dates in this article but I don’t know if I see all of them middle to heart. There is some validity but I’ll take maintain opinion until I look into it further. Good article , thanks and we wish more! Added to FeedBurner as nicely
Thanks
It’s nice that you pointed out how photochemistry is the study of chemical reactions caused by the absorption of light. I saw a science show on the TV earlier this morning and I got intrigued by the topic of photochemistry. I heard there are even photo chemistry kits being sold nowadays, and that is quite interesting.
Thank you sir for your reply