Colligative properties | Relative lowering of Vapour Pressure | Elevation in boiling point | Depression in freezing point | Osmotic pressure
Four (4) colligative properties
Colligative property-1
Relative lowering of Vapour Pressure Raoult’s Law
When a non-volatile solute is dissolved in a solvent, the vapour pressure of the solvent is lowered.
According, to Raoult’s law, the partial vapour pressure ‘P’ of a constituent of a liquid solution is equal to the product of its mole fraction ‘X1’ and its vapour pressure in the pure state.
i.e. P = X1Po ….(1)
Where, X1 = Mole fraction of solvent
Po = the vapour pressure of pure solvent
P = the vapour pressure of the solvent above a give solution.
Since X1 in any solution is less than unity, ‘P’ must be less than ‘Po’
OR
The dissolution of a solute in a solvent leads to lowering the vapour pressure of the latter below that of the pure solvent.
ΔP = Po – P
= Po – X1Po
= Po (1 – X1) ….(2)
For a solution X1 + X2 = 1
1 – X1 = X2
ΔP = PoX2
Where, X2 is mole fraction of solute.
Thus, the vapour pressure lowering of the solvent depends both on the vapour pressure of the solvent and the mole fraction of solute in solution.
The relative lowering of vapour pressure is given as,

Thus, the relative lowering of vapour pressure is equal to the mole fraction of the solute in the solution.
The relative lowering of vapour pressure is totally independent of the nature of solvent or solute but depends only on the amount of solute in a solution and hence, a colligative property.
Colligative property-2
Elevation in boiling point
Thermodynamically relation between the elevation in the boiling point of the solution and the molecular weight of non-volatile solute dissolved.
The boiling point of solvent is the temperature at which the vapour pressure of the solvent becomes equal to 1 atmosphere. When a non-volatile solute is added, the vapour pressure is lowered and the boiling point of the solution is increased. The difference in the boiling point of the solution and pure solvent is known as elevation in boiling point

If external pressure is Po then the solvent boils at To. The solution, however, attains this pressure Po only at temperature T.
The boiling elevation of the solution ΔTb is given as.
ΔTb = T – To (1)
By applying the Clausius Clapeyron equation, we have,


If ‘n1’ is no. of a mole of solvent & ‘n2’ is the number of moles of solute, then the mole fraction of solute is given as,


Where W1 & W2 are weights & M1 & M2 are the molecular weights of solvent & solute respectively.

Molality ‘m’ is the number of moles of solute dissolved in 1000 grams of the solvent.
Since W1 g of solvent contains W2/M2 moles of solute
Therefore 1000g will contain(W2/M2W1) x 1000 moles of solute which is molality,


Colligative property-3
Depression in freezing point
The relationship between depression in freezing point of the solution and molecular weight of Non-volatile solute dissolved.
The freezing point of the liquid is defined as the temperature at which vapour pressure of the liquid becomes equal to that of solids under one atmospheric pressure.
When a non-volatile solute is dissolved in a solvent the freezing point of the solvent is lowered. The difference between the two-freezing point is known as depression in freezing points.

If we plot a graph of vapour pressure Vs temperature it shows that ‘To’ is the freezing point of pure solvent & ‘T’ is the freezing point of the solution. Then, depression in the freezing point is given as
ΔTf = To – T …. (1)
Let ‘Ps’ correspond to vapour pressure of solid and pure liquid solvent at To and let ‘P’ be the vapour pressure of solid solvent and solution at temperature ‘T’. Let Po be the vapour pressure of the pure, super-cooled liquid at temp ‘T’.


If ‘n1’ is the number of moles of solvent 2 ‘n2’ is the number of moles of solute, then the mole fraction of solute. is given as
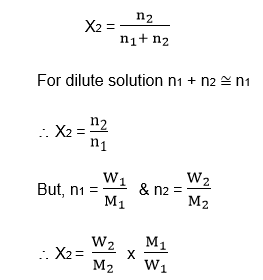
Where W1 & W2 are weights & M1 & M2 are molecular weights of solvent & solute respectively.
Substituting for X2 in equation (5) we get.


Osmosis
Osmosis is a phenomenon that refers to the movement or passage of solvent molecules from the pure solvent into a solution or from a dilute solution to a concentrated solution by a semipermeable membrane.
A semipermeable membrane is a membrane that is permeable to solvent molecules, but not to the solute particles
Colligative property-4
Osmotic pressure (π)
Osmotic pressure is the pressure that must be applied to the solution side to stop the inward flow of solvent.
Van’t Hoff equation

Consider the pure solvent in compartment I is at constant T and not subjected to any pressure change of solute addition.
Therefore d (𝓊A) Pure solvent = O (I)
i.e. d (GA) pure solvent = O (II)
where,
𝓊A = chemical potential
G = free energy/mol
The solvent in the solution of compartment (II) is at constant ‘T’ but subject to the addition of a solute and a ‘P’ change. The free energy changes due to of mole fraction ‘XA’ and pressure change ‘dp’ is given as.
dGA = VAdp + RTdℓnXA (III)
where,
XA = mole fraction of solvent
To maintain equilibrium,
dGA = O (IV)
Therefore VAdp = -RTdℓnXA (V)
Integrating eq. (V) between limit XA = 1 to XA pressure Pinitial and Pfinal respectively we get.
VA = (Pfinal – Pinitial) = – RTℓnXA (VI)
The excess of ‘P’ is osmotic pressure ‘π‘ and XA = 1 – XB for dilute solution.
XB << 1
Therefore ℓnXA @ – XB
Substituting in equation (VI)


π = CRT
where,
C = concentration in terms of moles per liter of solution (molarity of solution)
Equation (XI) is valid for dilute solution only.
Click the diagram below and read about “ROTATIONAL SPECTROSCOPY“

Molar mass of solute from osmosis
π = CRT
π = n2 /v . RT
n2 = no. of moles of solute
v = volume
n2 = w2/ m2
where,
w2 = weight of solute
m2 = molar mass of solute
π = .w2 /m2v . RT
m2 = w2 RT/ π v
By measuring ‘π’, the molar mass can be calculated.
Isotonic Pressure
When two solutions have the same osmotic pressure at a given temperature, they are said to be Isotonic pressure. They are of equimolar concentration.
Vant’s Hoff factor (i)
It is defined as the ratio of the experimental value of the colligative property to the calculated value of the property assuming ideal behaviors.

Ideal behavior = no association and dissociation.

Physical Significance
1. i = 1
When solute particles, neither dissociates and associates. E.g. Urea in water.
2. i < 1
When solute particles associate in solution. E.g. Acetic acid in benzene.
3. i > 1 When solute particles dissociate. Eg. NaCl in water
Abnormal Molar masses of solute
The expression of different collective properties is valid for a dilute solution. Hence it is possible to calculate the molecular weight of different solutes by experimentally determining the magnitude of the colligative property for a solution containing a known amount of solute. However, the molecular weight of the solute determined is found to be the same as the expected value for it is an abnormal value. The reason for such abnormality is as follows,
Association in Solution
The same solute like acetic acid, and benzoic acid when dissolving in a solvent like benzene, carbon tetrachloride exists as a dimer in solution. Such association reduces the number of particles in the solution. Thus the value of the colligative property which depends upon the number of particles is found to be less than the theoretical value. As a result molar mass calculated is much higher than expected.


Where,
n = no. Of particles produced during associating.
II. Vant’s Hoff factor (i) and degree of dissociation of solute in solution.
Let an electrolyte Axy undergoes dissociation which is represented as-
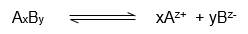
Where,
Z+ and Z- are charged on cation and anion respectively.
Therefore xz+ = yz- for electrical neutrality.
If ‘C’ molar concentration of electrolyte and ‘∝’ degree of dissociation, it follows that at equilibrium.
Concentration of undissociate Axby = (1 – ∝) c.
Concentration of dissociated ions = xc∝ + yc∝
Therefore Total concentration of solute particles = C [(1 – ∝) + x∝ + y∝]
Relation Between Vant’s Hoff factor (i) and degree of association of solute in solution
Let the association reaction of solute ‘A’ can be represented as


FAQs
What are the types of colligative properties? Or What are the 4 colligative properties?
There are total of 4 colligative properties:
1. Vapour pressure lowering, 2. Boiling point elevation, 3. Freezing point depression, and 4. Osmotic pressure.
Colligative properties depend on what?
Colligative property depends on the number of solute particles dissolved in the solution.
What is a simple definition of osmosis?
Osmosis is a phenomenon that refers to the movement or passage of solvent molecules from the pure solvent into a solution or from a dilute solution to a concentrated solution by a semipermeable membrane.

I know this if off topic but I’m looking into starting my own weblog and was wondering what all is needed to get setup? I’m assuming having a blog like yours would cost a pretty penny? I’m not very internet smart so I’m not 100 certain. Any recommendations or advice would be greatly appreciated. Appreciate it
I would like to thnkx for the efforts you have put in writing this blog. I am hoping the same high-grade blog post from you in the upcoming also. In fact your creative writing abilities has inspired me to get my own website now. Actually the blogging is spreading its wings fast. Your write up is a great example of it.
https://www.latesthairstylery.com Latest Hairstylery
Thank You Sir
The transparency of any issue is a good thing, and the residents would have easy access to all issues within their community. Are the bias reports going to show up in the police blotter or be reported publicly or will it take a FOIA request? Is it open to all bias and subjective interpretation?
Thank you very much…..
No importa, soy tonto lmao
After examine a couple of of the weblog posts on your website now, and I really like your manner of blogging. I bookmarked it to my bookmark web site listing and will likely be checking again soon. Pls check out my site as well and let me know what you think.
Algunas publicaciones de blog verdaderamente selectas en este sitio web, guardadas en fav.
you’re in point of fact a excellent webmaster. The web site loading speed is amazing. It kind of feels that you’re doing any distinctive trick. In addition, The contents are masterpiece. you’ve performed a wonderful activity on this topic!
I抳e learn a few good stuff here. Definitely price bookmarking for revisiting. I surprise how so much effort you put to make this sort of fantastic informative website.
Thank you very much sir
Gracias por compartir un artículo tan bueno con nosotros. Muchas gracias.
you are truly a just right webmaster. The website loading velocity is incredible. It kind of feels that you are doing any distinctive trick. In addition, The contents are masterwork. you’ve done a excellent job in this matter!
Hola! I’ve been reading your weblog for a while now and finally got the courage to go ahead and give you a shout out from Dallas Texas! Just wanted to say keep up the excellent job!
wonderful put up, very informative. I’m wondering why the opposite experts of this sector do not notice this. You must continue your writing. I am sure, you have a great readers’ base already!
Thank you dear
I am sure this piece of writing has touched all the internet viewers, its really really pleasant article
on building up new website.
Thank you very much.
That is very attention-grabbing, You’re an overly skilled blogger.
I’ve joined your feed and sit up for searching
for more of your great post. Additionally, I have shared your site in my social networks
Thank you very much.
I like the helpful information you provide in your articles. I抣l bookmark your blog and check again here regularly. I am quite sure I will learn plenty of new stuff right here! Best of luck for the next!
Thank you dear
I’m truly enjoying the design and layout of your blog.
It’s a very easy on the eyes which makes it much more pleasant for me to come here
and visit more often. Did you hire out a developer to create your theme?
Great work!
I seriously love your website.. Excellent colors & theme.
Did you develop this web site yourself? Please
reply back as I’m attempting to create my own website and want to learn where you got
this from or exactly what the theme is named.
Kudos!
Thank you dear
Wow, fantastic weblog format! How long have you been running a blog for? you make running a blog look easy. The whole look of your site is magnificent, as neatly as the content!
Thank you dear
Soy un poco novato con Jaaxy pero lo he usado lo suficiente para saber el valor de esta gran herramienta de palabras clave. Tu crítica es un gran recurso para alguien como yo con experiencia limitada en el uso de la herramienta. Aprendí una gran crítica
Thank you dear
Thanks for this glorious article. One more thing to mention is that nearly all digital cameras are available equipped with a new zoom lens that enables more or less of that scene for being included by means of ‘zooming’ in and out. These types of changes in the aim length are usually reflected inside the viewfinder and on significant display screen at the back of the actual camera.
Thank you dear
Thanks for your post. I would also like to say that the health insurance broker also works well with the benefit of the coordinators of the group insurance policy. The health broker is given an index of benefits wanted by a person or a group coordinator. What a broker may is try to find individuals or coordinators that best go with those demands. Then he presents his tips and if both sides agree, this broker formulates an agreement between the 2 parties.
Thank you dear
One thing is that when you’re searching for a student loan you may find that you will want a cosigner. There are many cases where this is true because you will find that you do not have a past credit rating so the lender will require that you have someone cosign the credit for you. Interesting post.
Thank you…
I have figured out some new issues from your web site about pcs. Another thing I’ve always believed is that computers have become a product that each household must have for several reasons. They provide convenient ways to organize homes, pay bills, shop, study, pay attention to music as well as watch television shows. An innovative way to complete every one of these tasks is to use a notebook computer. These pc’s are portable ones, small, potent and portable.
Thank you…
Can I just say what a relief to find someone who actually knows what theyre talking about on the internet. You definitely know how to bring an issue to light and make it important. More people need to read this and understand this side of the story. I cant believe youre not more popular because you definitely have the gift.
Valuable information. Lucky me I found your site by accident, and I am shocked why this accident did not happened earlier! I bookmarked it.
thank you very much dear
What i do not realize is actually how you’re no longer actually a lot more smartly-preferred than you may be now. You are very intelligent. You know thus considerably in terms of this topic, made me individually believe it from numerous numerous angles. Its like women and men aren’t interested unless it¦s one thing to accomplish with Woman gaga! Your own stuffs excellent. At all times maintain it up!
thank you very much dear
Very nice style and wonderful articles, practically nothing else we want : D.
thank you very much dear