What is Rotational spectroscopy or microwave spectroscopy?
Rotational spectroscopy which is also called microwave spectroscopy provides information about the absorption or emission of electromagnetic radiation typically in the microwave region of the electromagnetic spectrum.
Heteronuclear diatomic molecules are microwave active.
(i) A Molecule, to give rotational spectra should possess a permanent dipole moment.
(ii) Heteronuclear diatomic molecules possess a permanent dipole moment. This dipole moment gives rotational spectra.
(iii) If the energy supplied is small enough during the rotation of molecules, the bond length of the molecule remains unchanged.
(iv) The amount of energy required to change the rotational energy level is of the order of 0.05 ev ( 2.5×10-4 m) which corresponds to the energy of radiation in the microwave region.
(v) During rotation, these molecules generate an electric field that interacts with the microwave radiations giving rise to a spectrum.
(vi) This absorption of radiation take place in the infrared or microwave region of the electromagnetic spectrum
(vii) Due to the above reasons, Heteronuclear diatomic molecules are microwave-active, and Rotational spectra are known as Microwave spectra.
(viii) Example: HCl, HBr, and CO are microwave active.
Expression for the moment of inertia in a simple diatomic molecule.
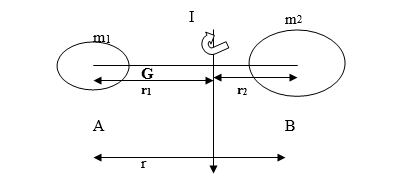
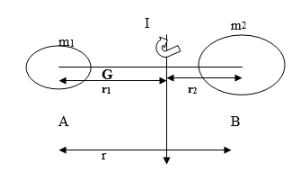
Consider a simple diatomic rigid polar molecule of masses ‘m1’ and ‘m2’ separated by an internuclear distance ‘r’.
-Let r1 and r2be the distances of these atoms from the center of gravity (G), about which the molecules rotate.
-Moment of inertia of a particle of mass ’m’ revolving around a fixed point at a distance ‘r’ is given by ‘mr2’.
-For a diatomic molecule, the total moment of Inertia (I) is given by,
I = m1r12+ m2r22………………………………….…….i)
Since the system is balanced, the moments of both atoms are equal i.e.
m1r1 =m2r2………………………………..……….… .ii)
On substituting values from eq (ii) in eq (i), we get
I = m2r2r1+ m1r1r2
I = r1r2 (m1 + m2) ……………………………….…..(iii)
But, r = r1 +r2
r= r1 + m1r1 / m2 …………………..(from eq ii)
r = r1(1 + m1/m2 )
r = r1(m1+ m2 /m2)
Therefore r1= m2/m1+m2 . r ……………………………… (iv)
Similarly r2 = m1/m1+m2 . r …..………..……………… (v)
On substituting the values of r1 and r2 in equation (iii) we get,
I = r ( m2/ m1+m2 ) × r (m2/m1+m2) × (m1 + m2)
Therefore I = m1m2/ m1+m2 . r2 ……………….. eq (vi)
The term m1m2/m1+m2 = µ i.e. reduced mass of the system
Therefore I =µ r2
The frequency separation (∆v) between two successive lines in the rotational spectrum is 2B i.e ∆ v = 2B.
The rotational spectrum of a diatomic molecule consists of equally spaced lines.
The equation for the frequency separation of the lines in the rotational spectrum of a diatomic molecule.
The rotational energy EJ of a diatomic molecule is given by,

Where, I =moment of inertia of the molecule
h =Planck s constant
J =rotational quantum number which can have value 0,1,2,3……
Consider a rotational transition from a rotational energy level J to another level (J1)

(c) Now change its rotation energy (∆E) is given as,
∆E = EJ – EJ’

But accordingly, to the selection rule, ∆J=+1
i.e. ∆J= J-J’= +1
Therefore J’= J-1
By substituting the equation of J’ in eq (iii) we get,

(d) According to quantum theory, energy changes are quantized by the equation,
ΔE = hυ = hῡc …………… ………………….…. ..(v)

Therefore ῡ = 2BJ
Where ῡ = frequency in wavenumbers of lines in the rotational spectrum.
(e) Thus, from eq(vi), the frequency of lines in the rotational spectrum can be found.
(i) When J=1, i.e for the transition from J=0 to J=1 ( Transition from ground state J=0 to the first energy level J=1 takes place)
Therefore ῡ 1 = 2Bm-1
(ii) When J=2, i.e for transition from J=1 to J=2 ( Transition from the first energy level J=1 to second energy level J=2 takes place )
ῡ 2 = 4Bm-1
(ii) When J=3, i.e for transition from J=2 to J=3
ῡ3 = 6Bm-1
(f) The frequency separation between consecutive lines will be,
∆ ῡ = ῡ2 – ῡ1 =4B – 2B = 2B
∆ ῡ = ῡ3 – ῡ2 =6B – 4B = 2B & so on . Thus the rotational spectrum of a diatomic molecule consists of a series of equidistance lines with the separation of 2B.

Thus, the frequency difference between two successive lines in the rotational spectrum is 2B.
(g) From the Moment of Inertia (I) the equilibrium distance ‘r’ or bond length can be calculated

Where m1 and m2 are masses of atoms in a diatomic molecule.
Diagram showing different rotational energy levels with permitted rotational transition in a domestic molecule
The nature of the rotational spectra:-
- The spectral lines are present in the microwave region.
- The spectrum consists of equally spaced lines.
- The spacing between any two successive lines is constant and is equal to 2B0m-1.
- At room temperature, the rotational energy levels up to J = 5 are occupied by the molecules. Hence, all the transitions from J1 = 0 to J1= 5 will be observed.

The effect of isotopic substitution on the rotational energy levels and rotational spectrum level of a diatomic molecule.
(a) The Presence of isotopes in a molecule will bring about a considerable change in the reduced mass (µ) of the molecules. However, the intermolecular distance (r) and geometry of the molecule remain unchanged. As reduced mass changes, it will bring about a change in the moment of inertia (I) and Rotational Constant (B).
(b) In the Relation B = h/8π2Ic, the rotational constant B is inversely proportional to the moment of inertia.
(c) Therefore, the heavier isotopic molecule having a larger moment of Inertia will have a small value of B. As a result, the following frequency separation of successive lines which depends on B will be smaller for the heavier isotopic molecule than for the lighter isotopic molecule.
(d) These small differences in the frequency separation have been utilized to determine the isotopic masses.
(e) Consider two isotopic molecules A and B. Let IA and IB be their moment of inertia and πA and πB be their reduced masses. The rotational constants BA and BB will be given by.

This relationship helps to calculate the abundance of isotopes by knowing the shifts in the spectral lines with the isotopic effect.
Microwave spectroscopy instrumentation
A typical microwave spectrometer consists of the following components,
a. The source and Monochromator
b. The Beam Direction
c. Sample and Sample Space
d. Detector
e. Recorder
Limitations of Rotational Spectra
The limitations of Rotational Spectra are as follows,
- Heteronuclear molecules and groups which have a permanent dipole moment alone give rotational spectra. eg. HCl, HCN, CH3Cl, etc.
- Homonuclear molecules and groups which do not give a permanent dipole moment do not give rotational spectra and thus cannot be studied by using rotational spectroscopy. eg. H2, N2, O2, etc.
- Similarly, linear and non-linear polyatomic molecules cannot be studied by using rotational spectroscopy as they do not show rotational spectra. In the case of polyatomic molecules, a single moment of Inertia will not give a complete molecular structure
- The substances under study should be in the gaseous condition for the free rotation of molecules to take place. In solids and liquids, strong intermolecular forces of attraction do not allow the free rotation of molecules. Hence, the substances in solid and liquid conditions cannot be studied.
FAQs
What do you mean by rotational spectra?
Rotational spectroscopy which is also called microwave spectroscopy provides information about the absorption or emission of electromagnetic radiation typically in the microwave region of the electromagnetic spectrum.
What type of molecules gives rotational spectra? or Which molecule will show rotational spectra?
Heteronuclear di-atomic molecules show rotational spectroscopy.
Rotational spectra are observed in which region
Rotational spectroscopy is observed in the microwave region. Hence it is also called microwave spectroscopy.
What is microwave plasma atomic emission spectroscopy?

Microwave plasma atomic emission spectroscopy is an atomic emission technique. in which an atom of a specific element is excited to its upper state, which emits light in a characteristic pattern of its wavelengths recorded as an emission spectrum, as it returns to the ground state by emission of characteristic wavelength.
Microwave plasma atomic emission spectroscopy (MP-AES) is one of the best models available in the market by Agilent Technologies. Agilent is a global leader in life sciences, diagnostics, and applied markets, recognized for uncompromising integrity.
I simply could not leave your web site before suggesting that I really loved the usual information an individual supply for your guests? Is gonna be again regularly in order to check out new posts
Thank you….
I used to be recommended this website by my cousin. I’m not sure whether or not this submit is written through him as no one else understand such specified about my trouble. You are amazing! Thank you!
Good post. I learn something tougher on totally different blogs everyday. It’s going to all the time be stimulating to learn content from different writers and apply somewhat something from their store. I抎 prefer to make use of some with the content material on my weblog whether you don抰 mind. Natually I抣l give you a hyperlink in your internet blog. Thanks for sharing.
I like what you guys are up also. Such smart work and reporting! Carry on the excellent works guys I have incorporated you guys to my blogroll. I think it’ll improve the value of my site 🙂
Thank you….
I have been browsing online more than three hours today, but I never found any attention-grabbing article like yours. It抯 beautiful price sufficient for me. In my opinion, if all website owners and bloggers made excellent content as you probably did, the net shall be a lot more helpful than ever before.
I’ve observed that in the world the present day, video games are classified as the latest trend with kids of all ages. Often times it may be difficult to drag your children away from the activities. If you want the very best of both worlds, there are many educational video games for kids. Thanks for your post.
Thank you….
Needed to send you that tiny note just to thank you so much over again considering the exceptional things you have shared on this site. This is so remarkably open-handed of you in giving without restraint what exactly numerous people would have sold for an e book to generate some profit for themselves, primarily given that you might have done it in the event you wanted. Those ideas additionally served to become a great way to understand that someone else have the identical keenness similar to mine to understand a good deal more in respect of this problem. I am certain there are some more fun periods in the future for folks who read through your site.
I have been browsing online more than 2 hours today, yet I never found any interesting article like yours.
It’s pretty worth enough for me. In my view, if all website
owners and bloggers made good content as you did, the internet will be a lot more useful than ever before.
It’s the best time to make some plans for the future and it is time
to be happy. I have read this post and if I could I want to suggest you few interesting things or tips.
Maybe you could write next articles referring to this article.
I want to read more things about it!
Hi to every one, for the reason that I am in fact eager
of reading this weblog’s post to be updated on a regular
basis. It includes nice stuff.
I’ve really noticed that repairing credit activity needs to be conducted with techniques. If not, you will probably find yourself causing harm to your position. In order to reach your goals in fixing to your credit rating you have to make sure that from this moment in time you pay your entire monthly dues promptly prior to their scheduled date. It really is significant on the grounds that by not really accomplishing that area, all other moves that you will decide on to improve your credit ranking will not be effective. Thanks for giving your tips.
I am usually to blogging and i really appreciate your content. The article has actually peaks my interest. I’m going to bookmark your web site and preserve checking for brand new information.
Excellent beat ! I would like to apprentice while you amend your website, how could i subscribe
for a blog web site? The account helped me a acceptable deal.
I had been tiny bit acquainted of this your broadcast
offered bright clear idea
I like what you guys tend to be up too. This type of clever work and exposure! Keep up the awesome works guys I’ve incorporated you guys to my blogroll.
Howdy very nice website!! Man .. Excellent .. Amazing ..
I’ll bookmark your website and take the feeds additionally?
I am glad to search out a lot of useful info
right here within the submit, we want work out
extra techniques on this regard, thank you for sharing.
. . . . .
Today, I went to the beach with my children. I found a sea shell and gave it to my 4 year old daughter and said “You can hear the ocean if you put this to your ear.” She put the shell to her ear and
screamed. There was a hermit crab inside and it
pinched her ear. She never wants to go back! LoL I know this is entirely off
topic but I had to tell someone!
Thank you dear
Woah! I’m really digging the template/theme of this site.
It’s simple, yet effective. A lot of times it’s very difficult to get
that “perfect balance” between superb usability and appearance.
I must say you have done a very good job with this. Additionally,
the blog loads very quick for me on Internet explorer. Exceptional Blog!
Thank you dear
This post will help the internet people for setting up new
blog or even a blog from start to end.
Thank you dear
Hi, I check your blogs daily. Your humoristic style is awesome, keep it up!
Thank you dear
I think this is one of the most important information for me.
And i am glad reading your article. But wanna remark on few general things,
The web site style is perfect, the articles is really excellent :
D. Good job, cheers
Thank you dear
I’m really enjoying the design and layout of your blog. It’s a very easy on the eyes which makes it much more pleasant for me to come here and visit more often. Did you hire out a designer to create your theme? Excellent work!
Thank you a bunch for sharing this with all folks you actually recognise what you’re speaking about! Bookmarked. Please also visit my site =). We can have a hyperlink alternate agreement between us!
I conceive this web site has some rattling superb information for everyone. “The expert at anything was once a beginner.” by Hayes.
Thank you
Thanks for the a new challenge you have revealed in your article. One thing I’d prefer to reply to is that FSBO associations are built eventually. By launching yourself to owners the first end of the week their FSBO can be announced, prior to a masses start out calling on Thursday, you make a good link. By giving them methods, educational products, free accounts, and forms, you become an ally. If you take a personal interest in them and their situation, you produce a solid link that, oftentimes, pays off in the event the owners opt with a representative they know and also trust — preferably you.
Thank you dear
Good write-up, I¦m normal visitor of one¦s website, maintain up the nice operate, and It’s going to be a regular visitor for a long time.
Thank you very much
Wow, this paragraph is nice, my sister is analyzing such things, thus
I am going to tell her.
Thank you dear
I am no longer sure the place you’re getting your information, but great topic. I must spend a while learning much more or understanding more. Thank you for excellent info I was on the lookout for this information for my mission.
thank you dear
I am glad to be a visitant of this sodding site! , regards for this rare info ! .
thank you dear
Men and women are almost always concerned about the long run and have invested heavily to make sure that tomorrow is really as good as can be. Many individuals even become involved in so many different methods, strategies or a name change to always make sure that they have a bright future.
Pretty nice post. I just stumbled upon your weblog and wanted to say that I’ve really enjoyed browsing your blog posts. After all I will be subscribing to your rss feed and I hope you write again very soon!
thank you dear
Have you ever considered about adding a little bit more than just your articles? I mean, what you say is valuable and everything. However think of if you added some great photos or video clips to give your posts more, “pop”! Your content is excellent but with images and video clips, this site could undeniably be one of the best in its field. Awesome blog!
Thank you very much for your suggestion. I will definitely do this improvement in upcoming blogs
I think other website proprietors should take this website as an model, very clean and excellent user pleasant design.
Thank you very much sir. Daily I am getting lots of good comments from reader. Hence some times it becomes very difficult to reply all on same day. Once again thank you very much.
Major thankies for the blog.Really looking forward to read more. Really Great.Loading…
Thank you very much sir. Plz forward this link to others also.
There are some fascinating points in time in this article however I don’t know if I see all of them center to heart. There may be some validity but I will take maintain opinion until I look into it further. Good article , thanks and we want extra! Added to FeedBurner as properly