Introduction to Vibrational Spectroscopy
- The rotational spectra of a diatomic molecule were considered with rigid bond, but the Co – valent bond is not perfectly rigid, it is somewhat flexible this flexibility gives rise to one more type of motion to the molecule which is vibrational motion.
- For vibrational motion absorption takes place in the IR region. After absorbing radiation in the IR region molecule vibrates at many rates of vibrations giving rise to closely packed absorption bands called “ IR- absorption spectra”.
- Various bands correspond to characteristic functional groups and bands present in a molecule. This IR spectroscopy is the most powerful technique to identify the chemical compounds OR IR spectra provide information about the nature of the bond and the structure of the molecule.
- The diatomic molecular system is stable and the atoms are separated by a distance known as bond length or internuclear distance.
- The stability of the molecule is due to two opposite forces,
- Force of repulsion between electrons of both the atom and nucleus of both the atoms.
2. Force of attraction between the electron of one atom and the nucleus of another atom and vise-versa.
- When these two forces are counterbalanced at a certain distance it gives the stable molecular system the change in energy of the atoms in a molecule is created by the absorption of IR radiations.
The equilibrium distance is disturbed and the atoms will experience vibrations. These energy changes are observed in the form of vibrational spectra.
Modes of vibrations
Various types of vibrations in IR spectroscopy
There are two types of fundamental vibrations for a molecule.
- Stretching vibration
- Bending vibration
Stretching vibration requires more energy than bending vibration
1) Stretching vibration: –
In these vibrations, the distance between the two atoms increases or decreases, but the atoms remain in the same bond axis. It is of two types
a. Symmetrical Scratching

In this stretching, the movement of atoms with respect to the central atom in a molecule is in the same direction.
b. Unsymmetrical Stretching: –
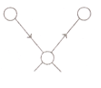
In this stretching Vibration, one atom approaches the central atom while the other departs from it.
2) Bending Vibration: –
Bending Vibrations are of four types.
a) Scissoring: –
In the Scissoring type two atoms move in opposite directions.
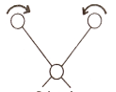
b) Rocking: –
In the Rocking type, the movement of atoms takes place in the same direction.

c) Wagging: –
In the Wagging type two atoms move either up or down the plane with respect atom.
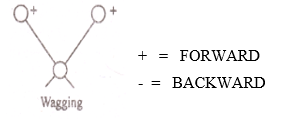
d) Twisting: –
In this type, one of the atoms moves up the plane while the other moves down the plane with respect to the central atom.

Examples
Introduction to infrared spectra of simple molecules:-
1. CO2 :- It is a linear triatomic molecule. Therefore, modes of vibration are
3n – 5, n = 3,
3n – 5 = 4. Two modes of vibrations are scratching and two are bending (asymmetric scratching).
In the case of CO2, the symmetrical stretching O =C= O does not involve any change in dipole moment and I.R. is Inactive.
In asymmetric stretching, one bond is stretched and the other is compressed.
O =C= O this produces the dipole moment of the molecule with the asymmetric stretching is I.R. Active. Two bending are identical and differ only in one direction.
a) O =C= O b) O =C= O
2. H2O: -It is a triatomic non-linear molecule. The modes of vibration are 3n – 6 = 3, n=3.
Two stretching and one bending mode of vibration. All are IR active.
Zero–Point Energy
Zero – Point Energy represents the lowest vibrational energy levels in a molecule. The Zero – Point Energy ‘ Eo’ of the molecule can be obtained from the equation,
Eo = (0 + ½)hc = ½ hc
It implies that the molecule is always vibrating and is never at rest even at absolute zero temperature when transitional and rotational motions are absent.
Thus, from the above equation, it is seen that this zero point energy depends on the vibrational frequency (ŵ) of the molecule and hence on the strength of the chemical and the atomic masses.
Force Constant.
The Force Constant (k) of a bond is a measure of the rigidity or stiffness of a bond i.e., the force required to stretch or compress a bond
The greater the value of the force constant strong is the bond and the higher the stability of the bond.
Vibrational or IR spectroscopy offers a convenient and reliable tool to determine the force constants of different types of bonds in the molecule.
Bond energy is the energy required to break a bond. Hence, the force constant can be directly related to the bond energy of a bond.
The wave number of the radiation absorbed for vibration is given by,
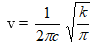
v =1 / 4 π2 c2 . (k/π)
Therefore K = 4π2 c2 π v2
Where π = reduced mass
v= wave number
The unit of force constant ‘k’ is Newton’s per meter (N.M-1)
Group Frequencies
The most practical use of IR spectra is in the field of organic chemistry. In a molecule, the bonds of the groups have definite vibrational frequencies and they are not affected by the rest of the molecule. Such frequencies are known as Group Frequencies.
Group Frequencies lie above and below the fingerprint region.
Groups with light atoms like –CH3, –OH,–CN, –C=O, etc. absorb above 1400cm-1
whereas the groups containing heavy atoms absorb below 700 cm-1
Vibration – Rotation Spectra
Vibrating Rotor:-
As changes in both rotational and vibrational energy take place, the expression for the energy of a vibrating rotor should include both expressions for the vibrational as well as rotational energy.
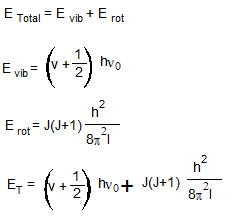
Selection Rule:-
- When radiation is absorbed or radiated by a molecule, it is found theoretically as well as experimentally transition occurs between certain energy levels as a result of what is known as the selection rule.
- For harmonic oscillator –rigid rotor, these require that vibrational quantum number ‘v’ and rotational quantum number J.
- Thus, under condition, if the spectral lines obtained are clumped together in what is known as R-branch lines and when transitions form another clump in the spectrum known as P-branch lines.
- Vibrational Energy level:-
The vibrational energy is quantized. The expression for the vibrational energy of a molecule is,
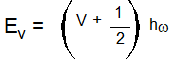
Where ⱳ is the frequency of oscillation,
v is the vibrational quantum number ( V = 0, 1,2,3……)
By assigning different values to the vibrational quantum number different energy values of the vibrational energy can be obtained.
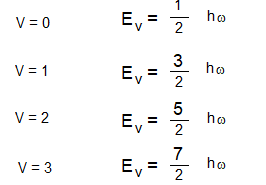
The Vibrational energy level diagram of the molecule is shown above.
P and R branches with reference to IR spectra
When a molecule behaves as a harmonic or inharmonic oscillator there is a simultaneous change in rotational energies. This combined spectrum is called a vibrational, rotational spectrum. Vibrational energy changes are 100 times greater or more than rotational energy changes. They are considered independent & there is no interaction between them. It is given by,

Where B is the rotational constant.
For harmonic oscillations (v – v’) = 1

That is no Peak.
This frequency is known as the bond center which gives a flat curve.
The spectral line corresponding to this is called P-branch and R-branch.
This vibrational–rotational spectrum is called P – R spectra which is equally spaced with a flat curve or a gap in the center. Eg HBr
Frequencies of the fundamental first and second overtone bands are approximately in the ratio 1:2:3.
(a) Molecules whose vibration satisfies the selection rule ∆v = are called Harmonic. In this case, the stretching and bending of the bond is small and they absorb little energy for vibration.
(b) The molecules which can absorb large amounts of energy and show distortions are called Anharmonic oscillators. (c) The vibrational energy anharmonic molecule is given by the equation,

Where, v = vibrational quantum number having values 0,1,2……
h = Planck‘s constant.
= oscillatory frequency in wavenumber
But, A = (v + ½)2hcѿx
Where x = Anharmonicity constant.
Therefore, on substituting valve of A in eq (i) becomes
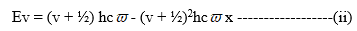
(d) Let the vibrational quantum number change from v = 0 to v=1. The energy change corresponding to this transition can be given as,

This absorption band is known as the fundamental band or first harmonic band.
(e) When the transition occurs from V = 0to V = 2, the frequency of spectral lines is given by,
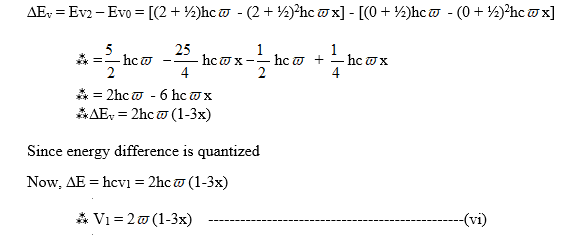
This is known as the first overtone or second harmonic band
(f) Similarly, The frequency of spectral lines of transition from V0 to V3 is given by,

This is known as the second overtone or third harmonic band.
(g) Therefore It is observed, the frequencies of eq (v), (vi) & (vii) i.e., fundamental, first overtone, and second overtone are in proportion,
ѿ (1-2x) : 2 ѿ(1-3x) : 3 ѿ(1-4x)
Here, x is small which can be neglected.
ѿ : 2 ѿ : 3 ѿ i.e. 1:2:3.
The frequencies of the fundamental first and second overtone bands are approximately in the ratio 1:2:3 And in terms of wavelength they are 1 :1/2: 1/3
FAQs
What do you mean by vibrational spectroscopy?
For vibrational motion absorption takes place in the IR region. After absorbing radiation in the IR region molecule vibrates at many rates of vibrations giving rise to closely packed absorption bands called “ IR- absorption spectra”.
Which spectroscopy is known as vibrational spectroscopy?
Infrared spectroscopy (IR spectroscopy) is known as vibrational spectroscopy.
Its like you read my mind! You appear to know a lot about this, like you wrote the book in it or something. I think that you could do with a few pics to drive the message home a little bit, but instead of that, this is wonderful blog. An excellent read. I’ll certainly be back.
Thank you a bunch for sharing this with all people you really realize
what you’re speaking approximately! Bookmarked.
Please also seek advice from my website =).
We will have a link alternate contract between us
Thank you very much.
If you desire to get a good deal from this article then you
have to apply these strategies to your won weblog.
Attractive component of content. I just stumbled upon your weblog and in accession capital to assert that I get in fact loved account your weblog posts. Anyway I will be subscribing for your augment or even I fulfillment you get right of entry to persistently fast.
Hello there! I know this is kinda off topic but I was
wondering if you knew where I could get a captcha plugin for
my comment form? I’m using the same blog platform as yours and
I’m having problems finding one? Thanks a lot!
What i don’t understood is in reality how you are not
actually much more well-liked than you might be right now.
You’re very intelligent. You understand therefore significantly with regards to this subject, made me personally imagine it from
a lot of varied angles. Its like women and men aren’t fascinated until
it’s something to do with Woman gaga! Your personal stuffs
outstanding. All the time handle it up!
What’s up colleagues, how is all, and what you desire to say
regarding this paragraph, in my view its really awesome designed
for me.
excellent post, very informative. I ponder why the other experts of this sector do not realize this. You must continue your writing. I am sure, you’ve a huge readers’ base already!
Nice post. I learn something more difficult on completely different blogs everyday. It should all the time be stimulating to read content material from other writers and apply a little something from their store. I抎 want to make use of some with the content on my weblog whether or not you don抰 mind. Natually I抣l provide you with a hyperlink on your net blog. Thanks for sharing.
Appreciate you sharing, great article post. Will read on…
My brother recommended I might like this blog. He was entirely right. This post actually made my day. You can not imagine just how much time I had spent for this information! Thanks!
Hello, i read your blog from time to time and i own a similar one and i was just curious if you get a lot of spam responses? If so how do you stop it, any plugin or anything you can advise? I get so much lately it’s driving me mad so any assistance is very much appreciated.
Thank you dear
Hi this is kind of of off topic but I was wondering if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding knowledge so I wanted to get guidance from someone with experience. Any help would be enormously appreciated!
Please let me know if you’re looking for a author for your site. You have some really good articles and I believe I would be a good asset. If you ever want to take some of the load off, I’d love to write some articles for your blog in exchange for a link back to mine. Please send me an email if interested. Kudos!
Ok
thanks..
I will let you know
Hello, Neat post. There’s an issue together with your web site in web explorer, may test this… IE nonetheless is the marketplace leader and a huge part of folks will miss your wonderful writing due to this problem.
ok.thank you
hello!,I like your writing so much! share we communicate more about your post on AOL? I require a specialist on this area to solve my problem. Maybe that’s you! Looking forward to see you.
Sweet web site, super style and design, real clean and utilise friendly.
thank you very much dear
Very good written information. It will be valuable to anybody who utilizes it, as well as yours truly :). Keep up the good work – i will definitely read more posts.
thank you very much dear
I have not checked in here for some time because I thought it was getting boring, but the last few posts are good quality so I guess I will add you back to my daily bloglist. You deserve it my friend 🙂
thank you very much dear
Hi my loved one! I want to say that this article is amazing, great written and include approximately all important infos. I would like to see extra posts like this.
thank you very much dear
Your mode of explaining everything in this piece of writing is
really nice, all can without difficulty be aware of it,
Thanks a lot.
thank you very much dear
Someone necessarily assist to make seriously posts I’d state. That is the first time I frequented your website page and to this point? I amazed with the analysis you made to create this particular publish incredible. Excellent process!
Thank you sir for your comment.
I’d must verify with you here. Which isn’t something I often do! I enjoy studying a submit that will make individuals think. Additionally, thanks for allowing me to remark!
Thanks for review, many comments and suggestions.