Crystal Field Theory | CFT | Crystal Field Splitting in Octahedral complexes | Crystal Field Splitting in Tetrahedral complexes.
Crystal Field Theory or (CFT)
What is Crystal Field Theory or CFT?
The valence bond approach does not explain to us the Electronic spectra, Magnetic moments, and Reaction mechanisms of the complexes. Hence Crystal Field Theory was designed to overcome the limitation of valance Bond Theory (VBT). Crystal Field Theory (CFT) is commonly used for explaining the bonding in coordination complexes.
It explains many important properties of transition-metal complexes, including their colors, magnetism, structures, stability, and reactivity which were not explained by VBT. The central assumption of CFT is that metal-ligand interactions are purely electrostatic in nature. The interaction between metal ions and legends is the backbone of this theory.
Crystal Field Splitting (CFS)
Define Crystal field Splitting or CFS.
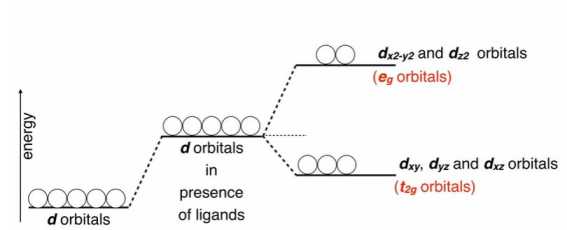
Splitting of the five degenerated orbitals of the free metal ion by the ligand field into two groups, having different energies is called Crystal field splitting or CFS.
Crystal Field Splitting in Octahedral complexes
Explain in brief crystal field splitting in the octahedral complexes.
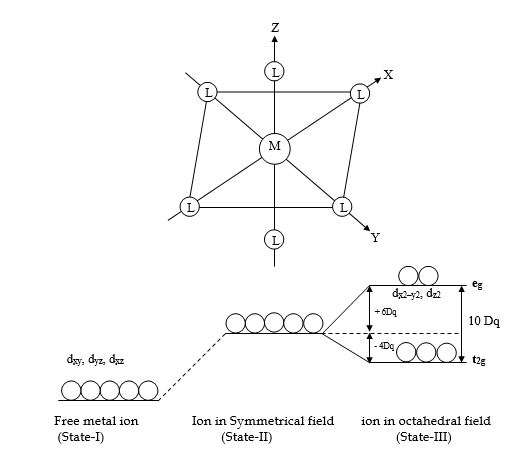
- In an octahedral complex, the metal ion is at the center of the regular octahedron and ligands are at the six corners of the octahedron along the X, Y, and Z axes.
- In free metal ions, all the five d-orbitals have the same energy i.e. they are degenerate (State-I). As the ligands approach the central metal ion, repulsion will take place between metal electrons and the negative electric field of ligands. This repulsion will raise the energy levels of d-orbitals. If all the ligands approaching metal ion are at an equal distance from each of the d-orbitals, then the energy of each d-orbital will increase by the same amount i.e. they will remain still degenerate (State-II). However, this is only a hypothetical situation.
- Because of different directional properties, the five d-orbitals will be repelled to different extents. Therefore their energies no longer remain the same but split up into two sets of orbitals called t2g and eg. The t2g orbitals include dxy, dyz, and dxz orbitals while “eg” orbitals include dx2-y2 and dz2 orbitals.
- ‘t2g’ orbitals, which lie in between the axes do not face the ligands directly and hence will experience less repulsion. Therefore ‘t2g’ orbitals will be lowered in energy by 4Dq relative to barycenter. ‘eg’ orbitals which lie along the axes, face the ligands directly and hence will experience more repulsions. Therefore ‘eg’ orbitals will be raised to a higher energy level by 6Dq relative to the barycenter.
- The difference in energy between the t2g and eg sets of d-orbitals is denoted by 10 Dq or ∆o and is called crystal field splitting energy in Octahedral complexes (State-III).
Weak and strong field ligands
What are Weak and strong field ligands?
Ligands that produce weak fields and cause a smaller degree of splitting of d-orbitals are called weak field ligands. Examples, are F-, OH-, and H2O. Ligands that produce a strong field and cause a larger degree of splitting of d-orbitals are called strong field ligands. Examples, are CN– and CO.
Crystal Field Stabilization Energy (CFSE)
What is Crystal Field Stabilization Energy? or What is CFSE?
The decrease in energy achieved by preferential filling of the lower energy d-levels is known as Crystal Field Stabilization Energy
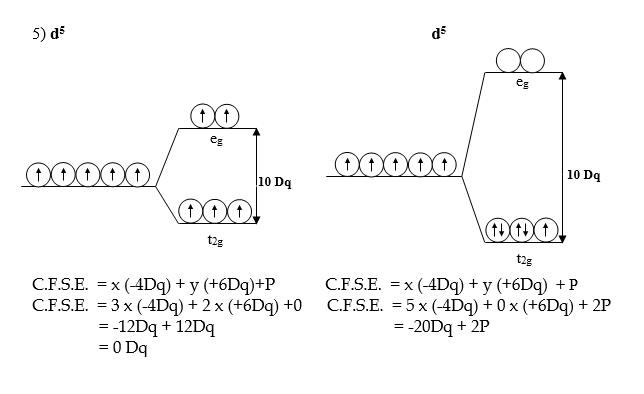
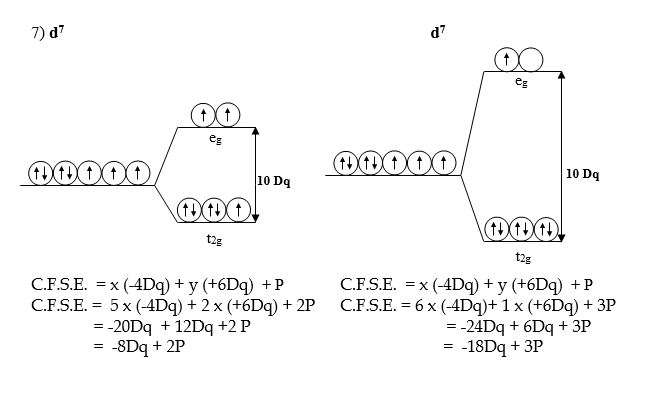
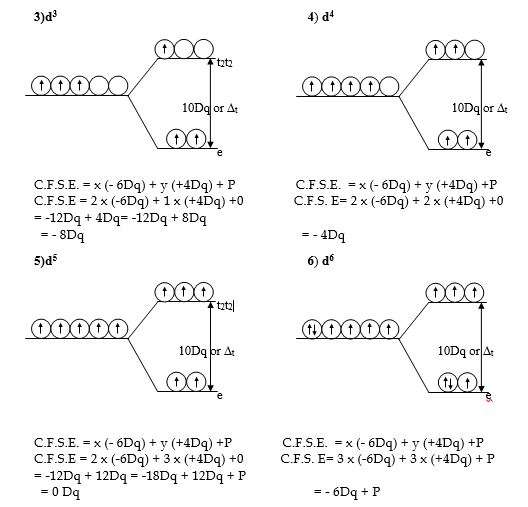
Crystal Field Stabilization Energy C.F.S.E. for the Octahedral complexes with d1 to d10 Configuration.
Crystal Field Stabilization Energy for the various configurations in the Octahedral field can be calculated by, CFSE formula:-
C.F.S.E. = x (-4Dq) + y (+6Dq) + P
Where,
x= number of electrons in t2g orbitals.
y = number of electrons in eg orbitals
P = Pairing energy






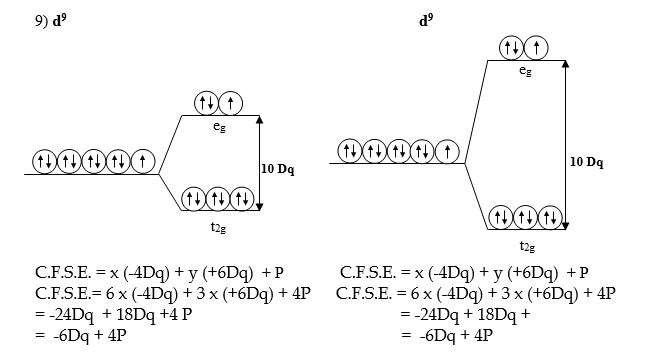

Crystal field splitting in tetrahedral complexes or splitting of d orbitals in tetrahedral complexes.
Describe Crystal field splitting of d orbital in tetrahedral complexes.
- In a tetrahedral complex, the metal ion is at the center of the regular tetrahedron and ligands are at the four alternate corners of the tetrahedron.
- In free metal ions, all the five d-orbitals have the same energy i.e. they are degenerate (State-I). As the ligands approach the central metal ion, repulsion will take place between metal electrons and the negative electric field of ligands. This repulsion will raise the energy levels of d-orbitals. If all the ligands approaching metal ion are at an equal distance from each of the d-orbitals, then the energy of each d-orbital will increase by the same amount i.e. they will remain degenerate (State-II). However, this is only a hypothetical situation.
- Because of different directional properties, the five d-orbitals will be repelled to different extents. Therefore their energies no longer remain the same but split up into two sets of orbitals called e and t2. The e orbitals include dx2-y2 and dz2orbitals while “t” orbitals include dxy, dyz, and dxz orbitals.
- ‘e’ orbitals, which lie along the axes do not face the ligands directly and hence will experience less repulsion. Therefore it will be lowered in energy by – 6Dq relative to barycenter. On the other hand, ‘t2’ orbitals which lie in between the axes, also do not face the ligands directly but are closer to ligands than ‘e’ orbitals and hence will experience more repulsions. Therefore it will be raised to a higher energy level by +4Dq relative to the barycenter.
- The angle between the ‘e’ orbital, the central metal, and the ligand is 540, 44’ which is half the tetrahedral angle. The angle between the ‘t2‘ orbital, the central metal, and the ligand is 350, 16’.
- The difference in energy between the two sets of d-orbitals is denoted by 10 Dq or ∆t and is called crystal field splitting energy in tetrahedral complexes (State-III).
- It is found that ∆the t value is always less than that of ∆o
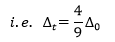
Comparison of ∆t with ∆o
The reasons for a smaller ∆t value compared to ∆o are as follows,
- In octahedral complexes, 6 ligands are involved while in tetrahedral complexes only 4. This resulted in a 33% decrease in the field strength of metal d-orbitals.
- In octahedral complexes, the ligands are situated exactly in direction of the dz2 and dx2-y2 orbital (‘eg’ orbitals). In tetrahedral complexes, the ligands are not situated at any of the d-orbitals but exert more influence on the “t2” orbitals than on the “e” orbitals.

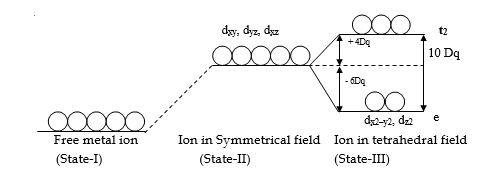
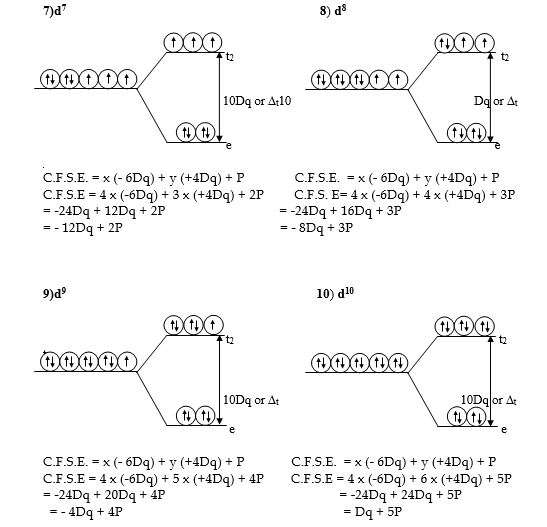
Crystal field stabilization energy C.F.S.E. for the tetrahedral complexes
What is Crystal Field Stabilization Energy? Calculate C.F.S.E. for the tetrahedral complexes with d1 to d10 Configuration.
The decrease in energy achieved by preferential filling of the lower energy d-levels is known as Crystal Field Stabilization Energy.
Crystal Field Stabilization Energy for the various configurations in the tetrahedral field can be calculated by the general formula,
C.F.S.E. = x (-6Dq) + y (+4Dq)
Where,
x = the number of electrons in e orbitals.
y = number of electrons in t2 orbitals.
P = Pairing energy
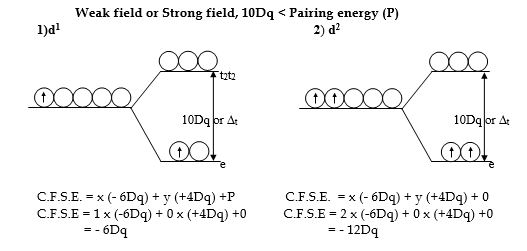
Tetrahedral complexes – high spin complexes
Most of the tetrahedral complexes are high-spin complexes
In tetrahedral complexes, the five degenerate metal ‘d’-orbitals split into two energy levels, the upper ‘t2’ and the lower ‘e’ level. The energy gap between ‘e’ and ‘t2‘ is denoted by ∆t.
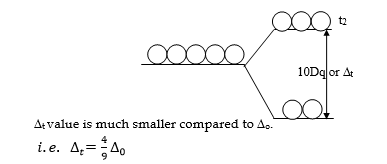
Since ∆t is much smaller compared to ∆o and ∆t < pairing energy(P), the electron prefers ‘t2’ orbitals rather than pairing up in ‘e’ orbitals in tetrahedral complexes. Hence most of the tetrahedral complexes are high-spin complexes.
Crystal field splitting in Square Planar complexes.
Define crystal field splitting. Explain in brief Crystal field splitting in Square Planar complexes.
Splitting of the five degenerated orbitals of the free metal ion by the ligand field into two groups, having different energies is called Crystal field splitting.
Crystal field splitting in square planar complexes
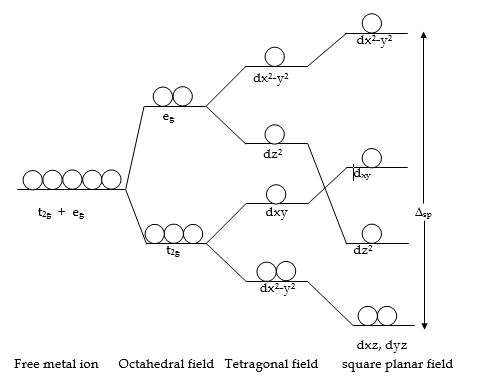
- In an octahedral complex, all ligands are at an equal distance from the central metal. If two trans ligands lying along Z-axis are slightly moved away from the central metal, then the distance between the central metal and trans ligands becomes more than that between the metal and the other ligands on XY plane. This will result in a tetragonally distorted octahedral structure.
- In the tetragonal structure, the metal d-orbitals, dz2, dxz, dyz with Z component will experience less repulsions, and the other two d-orbitals dx2-y2, dxy will experience more repulsions from the ligands than they do in an octahedral environment. Therefore, under the influence of ligands in a tetragonal complex, the energies of dz2, dxz, dyz are lowered and dx2-y2, dxy is raised. Therefore, the order of increasing energy of d-orbitals is as follows, dxz= dyz<dxy<dz2<dx2- y2
- A square planar complex can be derived from the tetragonal structure by removing completely the two trans ligands along Z-axis. In a square planar complex, there are only 4 ligands on the XY plane. The loss of two ligands on the Z-axis allows the remaining 4 ligands to move closer to the central metal ion destabilizing the dx2-y2 and dxy orbitals. Hence the d-orbitals of central metal involving Z component i.e. dz2, dxz, dyz are further lowered while the other two i.e. dx2-y2,dxy are further raised in energy.
Therefore the order of increasing energy of d-orbitals in a square planar complex is as follows,
dxz = dyz<dz2< dxy<dx2– y2.
In square planar complexes, the d-orbitals split into 4 levels. The energy difference between the lowest degenerate dxz, dyz pair, and the highest dx2-y2 is called Crystal field splitting energy and is denoted by ∆sp. The value of ∆sp is larger than ∆o. It has been found that ∆sp is about 1.3 times ∆o.

Factors affecting the Magnitude of 10Dq
What are the factors which affect the Magnitude of 10Dq or ∆o
The factors affecting the magnitude of 10 Dq or ∆o are as follows,
- The geometry of the complex:
The splitting of the d-orbitals in the octahedral complex is twice as strong as in the tetrahedral complex. This difference in the 10 Dq value is due to two factors.
- In octahedral complexes, 6 ligands are involved while in tetrahedral complexes only 4. This resulted in a 33% decrease in the field strength of metal d-orbitals.
- In octahedral complexes, the ligands are situated exactly in direction of dz2 and dx2-y2 orbital (‘eg’ orbitals). In tetrahedral complexes, the ligands are not situated at any of the d-orbitals but exert more influence on the “t2” orbitals than on the “e” orbitals.
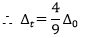
In square planar complexes, the value of ∆sp is about1.3 times ∆o
Please read How to treat wastewater Aerobic and Anaerobic treatment of water.
- Nature of the ligands
The value of 10 Dq for any given metal ion depends upon the ligand attached to it. The values of 10 Dq for Cr3+ complex with different ligands are as follows,
| Complex | 10 Dq or ∆0 [KJ mol-1] |
| [CrCl6]3- | 160.5 |
| [Cr(H2O)6]3+ | 213.1 |
| [Cr(NH3)6]3+ | 259.1 |
| [Cr(CN)6]3- | 314.1 |
It is clear that CN1- ligand produces more splitting and hence it is a strong ligand while Cl1- ligand produces less splitting and hence is a weak ligand.
- Charges on the Metal
The magnitude of D0 increases as the charge on the metal ions increases. A metal ion with a higher charge draws the ligands closer, and hence produces more splitting than an ion with a lower charge. e.g.
| Complex | 10 Dq or ∆0 [KJ mol-1] |
| [Cr(H2O)6]2+ | 166.1 |
| [Cr(H2O)6]3+ | 213.1 |
- Position of metal in the transition series.
The magnitude of D0 depends upon the position of the metal in the transition series, i.e., whether the metal is from the first, second, or third transition series involving 3d, 4d & 5d orbitals respectively. The value of 10Dq increases on descending down a group from the first to the third transition series.
| Complex | 10 Dq or ∆0 [KJ mol-1] |
| [Co(NH3)6]3+ | 274.9 |
| [Rh(NH3)6]3+ | 406.4 |
| [Ir(NH3)6]3+ | 489.9 |
This is explained on the basis that 4d orbitals in comparison to 3d orbitals are bigger in size and extend further into space. As a result, the 4d orbital can interact more strongly with the ligands and, therefore, the crystal field splitting is more. Similarly, 5d orbitals are still bigger than 4d orbitals and hence crystal field splitting is still greater.
Nephelauxetic Effect
Write a note on the Nephelauxetic Effect
a) It is found that the inter electron repulsions in the complexed metal ion,{[Fe(CN)63-]} are less than those in the free metal ion (Fe3+).
b) The decrease in the inter-electron repulsions in the complexed metal ion may be possibly due to the increase in the distance between the d-electrons. This increase in the distance can be attributed to an increase in the size of the d-orbitals. This expansion of the d-electron cloud could be partly due to the overlap of metal ion d-orbitals with the ligand orbitals. This effect of ligands causing the expansion of the d-electron cloud is known as the Nephelauxetic (cloud expanding) effect.
c) Depending upon the ability of the ligands to expand the d-electron cloud of metal ions, they are arranged in the form of a series called the Nephelauxetic series,
F–< H2O < NH3< C2O42-< en < NCS1-< Cl1-< CN1-< Br1-< I1- d) This series is independent of the central metal ion, like spectrochemical series. From the series, it is clear that F1- and I1- are at the two ends of the series. Therefore iodo complexes are more covalent than fluoro complexes. Thus, the Nephelauxetic effect provides evidence in support of covalent bonding.
Spectrochemical series
Write a note on the spectrochemical series
Magnitudes of crystal field splitting depend upon the nature of the ligands. Different ligands cause crystal field splitting to a different extents.
b) From the absorption spectra of the complexes of the same metal ion with different ligands, the value of 10Dq can be predicted. From the values of 10Dq, the ligands can be listed in the order of increasing capacity to cause splitting. The order is as follows,
I–< Br–< S2-< SCN–< Cl–< NO3–< F–<OH–< C2O42-< H2O < NCS–<NH3< en < dipy < Phen < NO2–< CN–< CO This arrangement of ligands in the order of increasing field strength is called Fajans-Tschida spectrochemical series because it is derived from spectral studies of the complexes. The ligands at the upper end of the series are called the weak field ligands and usually give high spin complexes, while the ligands at the lower end of the series are called the strong field ligands and usually give low spin complexes
FAQs
Q. What are High and Low spin complexes?
Complexes in which electrons remain unpaired to the maximum possible extent leaving a maximum number of unpaired electrons are called high-spin complexes. In a weak field, 10Dq is usually lesser than pairing energy, i.e. 10Dq < P. hence electrons remain unpaired and form high spin complexes.
Complexes in which electrons pair up to the maximum possible extent leaving a minimum number of unpaired electrons are called low-spin complexes. In a strong field, 10Dq is usually greater than pairing energy, i.e. 10Dq > P. Hence electrons pair up in the lower energy orbitals and form low spin complexes.
Q. What is the CFSE formula?
C.F.S.E. = x (-4Dq) + y (+6Dq) + P
Where,
x= number of electrons in t2g orbitals.
y = number of electrons in eg orbitals
P = Pairing energy
Q. What is 10Dq or ∆o
The energy difference between two groups of orbitals (t2g and eg) is called 10Dq or ∆o.
Q. What is CFSE full form?
Crystal Field Stabilization Energy.
Q. What is CFT full form?
Crystal Field Theory.

Nice post. I was checking constantly this weblog and I’m impressed! Very useful info particularly the ultimate part 🙂 I deal with such info a lot. I used to be seeking this certain info for a very long time. Thank you and best of luck.
Thank you dear
I am starting a business during the summer where I work with kids individually or during a camp. I was wondering how I could start a website for my clients parents to look at during the summer..
Right now it sounds like Movable Type is the preferred blogging platform out there right now. (from what I’ve read) Is that what you are using on your blog?
Thank you…
Thank you for the sensible critique. Me & my neighbor were just preparing to do some research on this. We got a grab a book from our area library but I think I learned more from this post. I am very glad to see such fantastic information being shared freely out there.
Thank you…
I truly appreciate this post. I have been looking all over for this! Thank goodness I found it on Bing. You have made my day! Thx again
Thank you…
As a Newbie, I am constantly browsing online for articles that can benefit me. Thank you
Thank you…
A lot of of the things you point out happens to be supprisingly appropriate and that makes me ponder the reason why I hadn’t looked at this in this light previously. Your article really did switch the light on for me as far as this particular subject matter goes. However at this time there is actually 1 factor I am not too comfortable with and while I attempt to reconcile that with the actual central idea of your issue, permit me observe what the rest of your subscribers have to say.Very well done.
Thank you
Hi there I am so excited I found your blog, I really found you by accident, while I was searching on Google for something else, Anyways I am here now and would just like to say thanks a lot for a tremendous post and a all round enjoyable blog (I also love the theme/design), I don’t have time to look over it all at the moment but I have saved it and also added your RSS feeds, so when I have time I will be back to read a lot more, Please do keep up the fantastic job.
Thank you
Fantastic website you have here but I was curious about if you
knew of any message boards that cover the
same topics discussed in this article? I’d really like to be
a part of community where I can get feed-back from other
experienced people that share the same interest.
If you have any recommendations, please let me know. Thanks!
I love reading through an article that will make men and women think. Also, thank you for permitting me to comment!
thank you
Hi, I found your post by mistake when i was searching google for this issue, I have to say your site is in actuality helpful I also love the theme, its amazing!. I dont have that much time to read all your post at the moment but I have bookmarked it and also add your RSS feeds. I will be back in a day or two. thanks for a great post.
thank you
I love looking through a post that can make men and women think. Also, many thanks for permitting me to comment!
Thank you very much dear
Lovely just what I was looking for.Thanks to the author for taking his clock time on this one.
Thank you very much dear
Very interesting information!Perfect just what I was looking for! “The medium is the message.” by Marshall McLuhan.
Thank you very much dear
I delight in, result in I discovered just what I was taking a look for. You have ended my four day lengthy hunt! God Bless you man. Have a nice day. Bye
Thank you very much dear
Excellent post. I was checking constantly this blog and I am impressed! Very useful information specially the last part 🙂 I care for such info a lot. I was looking for this certain information for a long time. Thank you and best of luck.
Thank you very very much. I am very much happy.
Thanks for this wonderful post but one thing I was wondering about crystal field splitting energy. Stronger the ligand higher is the crystal field splitting energy. But for all the d orbital in octahedral complex we find that for high spin complex formation the value of crystal field splitting energy is higher in value( taking the negative sign into account) than that of low spin complex formations. Shouldn’t it be the reverse? Please clarify. Thanks in advance.