Nanomaterials | Preparation of nanoparticles or Nanomaterials | Synthesis of nanoparticles.
Introduction
What are nanoparticles or Nanomaterials?
- Nano materials are the tiny particles of material that have size as in the range of 1-100 nm. The prefix Nano is a Greek word which means extremely small or dwarf. One nanometer is one billionth of a meter (1nm = 10-9 m). Nano particles are often crystalline.
- The transition from microparticles to nanoparticles can lead to a number of changes in physical properties such as colour, melting point, specific heat and magnetic property. For example, colour of solid gold is golden yellow but when the size of the gold particle is few nanometer in diameter then it will have either a blue or red colour depending upon the particle size.
- The two main factors that cause the properties of nanomaterials to differ significantly from those of other materials are i) increase in relative surface area (increase in area to volume ratio) and quantum effects. These enhance the properties of nanomaterials like reactivity, strength, electrical etc.
- In a material, as the particle size decreases, more number of atoms are found at the surface than inside. Hence, nanoparticles have a much greater surface area per unit mass compared to larger particles. Since catalytic reactions are surface based reactions, these reactions take place easily on nanomaterials.
- Quantum effects also affect optical, electrical and magnetic properties of nanomaterials. These enhanced properties are utilized in quantum dots, quantum well lasers etc
- Nanomaterials can be constructed by “topdown” technique. i.e., getting very small structures from larger particles or by “bottom up” technique i.e., self assembly of atoms or molecules one by one due to their natural properties.
- Nanomaterials can be viewed using scanning probe technique.
- Nanotechnology has wide applications in the field of pharmacy, information storage, refrigeration, catalysis, ceramics, semiconductors, etc.
Nanofilms and nano layers
What are Nanofilms and nanolayers What are the applications of Nanofilms and nanolayers?
- Nano films, Nanolayers and Surface Coatings: Nano films, Nanolayers and Surface Coatings are one dimensional nanomaterials. Thin films and engineered surfaces are used in fields of electronic device manufacture, chemistry and engineering. In silicon integrated circuit industry many devices depend upon thin films for their operations and the film thickness is carefully controlled to even atomic level.
- Thin films are created by one by one condensing the atomic or moleculer or ionic species of matter onto a substrate. Eg thin films of CuInSe2, Fe2O3. Engineered surfaces with larger surface area and specific reactivity are used very often in catalysts and fuel a cells.
Carbon Nanotubes
What are Nanotubes?
A nanotube is a nanoscale material that has a tube-like structure. Carbon nanotubes are nothing but composed of carbon atoms linked together in hexagonal shapes, with each carbon atom covalently bonded to three others having diameters as small as 1 nm and lengths up to several centimeters.
Nanotubes are of two types,
a) Carbon-nano tubes:
Carbon nanotubes are two-dimensional nanomaterials. They are either single-walled or multi-walled. They are a few nm in diameter and several mm – cm in length. Carbon nanotubes are mechanically very strong, flexible, and can conduct electricity. It is used in reinforced composites, sensors, nanoelectronics, etc.
b) Inorganic nanotubes:
Inorganic nanotubes like molybdenum disulfide were discovered after carbon nanotubes. These materials have excellent lubricating properties, resistance towards shock, catalytic reactivity, and high capacity for hydrogen and lithium storage. Nanotubes of TiO2 are being worked out in catalysis and energy storage.
Nanowires
Explain Nanowires.
Nanowires are two-dimensional nanomaterials. They are formed by self-assembly. Semiconductor nanowires made up of silicon, gallium nitride and indium phosphides have remarkable optical, electronic, and magnetic properties. Hence they have wide applications in electronic, optoelectronic devices, high-density data storage, etc. A nanowire transistor is recently designed for sensitive probing of the interior of cells.
Nano particles
Explain Nanoparticles:
- Nanoparticles are three dimensional nanomaterials. They have extremely small size i.e. less than 100 nm in diameter. The nanoparticles have transient behavior due to high diffusion rates and high typical concentration.
- Nanoparticles exist naturally as products of photochemical and volcanic activity and are created by algae and plants.
- Titanium dioxides and zinc oxide in nanoscale become transparent and is able to absorb and reflect UV radiation. Hence they are used in sunscreens.
- Nanoparticles are used in a small number of consumer products such as cosmetics and their special properties may have implications for their toxicity.
- Fullerenes, dendrimers and quantum dots (semiconductors) are some other useful forms of nanomaterials.
Optical properties of nano materials
Describe the optical properties of nanomaterials:
a) The optical spectra of single atoms show sharp absorption lines while bulk metals usually show wide absorption bands. When more and more atoms join together, lines split and bands develop. While when a large particle is made smaller, bands open and band gaps grow wider. The emission wavelength of small nanocrystals depends on their size.
b) According to the conduction or Drude model, a metal ion is surrounded by conduction or free electrons called Plasma. As electromagnetic radiation interacts with metal, the loosely held electrons called plasma start oscillating with a certain frequency and are known as plasmons.
c) UV or IR electromagnetic waves can penetrate metal particles up to the depth of ~50 nm. The surface plasmons contribute more to the photoelectric effect, in nanoparticles than in bulk material. When the frequency of incident radiation is the same as that of plasmon frequency, then resonance occurs and it is called surface plasmon resonance (SPR). The corresponding bands are called SPR bands.
d) SPR depends on particle size, shape, particle-particle interactions, and refractive index of the medium. In the case of bulk materials, these bands are propagative and in the case of nanoparticles, they are localized. Thus the color of the nanoparticles of Mg, Au, Ag, etc, are explained on the basis of SPR which is observed invisible.
e) In the case of semiconductor nanoparticles, valence and conduction bands are separated by a gap. This gap is different for different materials. The electromagnetic radiations, incident on the materials should be large enough to excite an electron from the valence band to the conduction band. With the decrease in particle size, the energy gap increases, more energy is required for the excitation of electrons, and radiations of shorter wavelength have to be absorbed.
Example: CdS semiconductor.
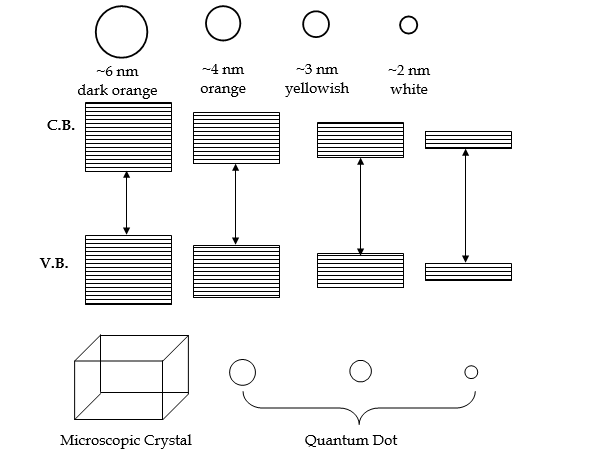
Semiconductor nanoparticles doped or undoped, show enhanced luminescence compared to their bulk counterparts. In nanomaterials, the energy gap can be tuned with the particle size. Hence, their luminescence can also be tuned to the desired wavelength.
Electrical properties of nano materials.
Describe the Electrical properties of nanomaterials.
i) Mechanical properties of materials are explained in terms of toughness, hardness, plastic deformation, stiffness, and ductility of the material.
ii) Mechanical properties depends upon the following factors,
- Types of bonds present between the atoms like ionic, covalent or metallic.
- Composition of the material and whether impurities like C, O, N, S, P are present or not.
- Point defects, grain boundaries, dislocations etc.
iii) Most of the metals are made up of small crystalline grains. When the material is stressed, boundaries between the grains decrease and do not allow defects to increase e.g., nanocrystalline nickel is as strong as hardened steel.
iv) Hardness is an important mechanical property of solid material. It is a measure of the ability of a material to resist local, plastic deformation. Various scales are used to measure hardness. The most commonly used scale is the Vicker Hardness scale (VHN) in which a small diamond is used as an indentor to scratch the material. The resulting impression or scratch is studied further with the help of a microscope.
v) Hardness of material depends on particle size. In a bulk material, it increases with an increase in size, but in nanoscale, the hardness of material increases with a decrease in particle size. For example, copper, in micrometer (mm) grain size, hardness increase with the increase in grain size. But in nanometer, the hardness increases with a decrease in particle size.
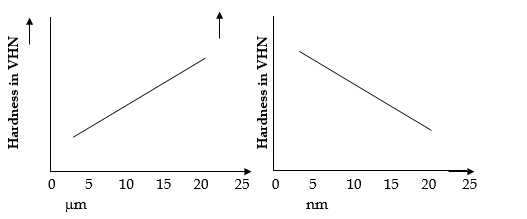
vi) In Metallic nanocrystalline materials, the elastic moduli get reduced dramatically. Ceramic materials are usually compacted and sintered using powder material. This helps in increasing their hardness. When nanoparticles of TiO2 are produced in powder form, less temperature is required to densify and acquire the hardness comparable to polycrystalline material.
Preparation of nanoparticles or Preparation of nanomaterials or Nanoparticle preparation
Explain the preparation of nanoparticles by the colloidal route method.
Colloidal route method
In a colloidal solution, the colloidal particles are usually charged. As soon as there are some charges on the particles, ions of opposite charge collect around them and form counter ions. The accumulation of counter ions leads to the formation of the electrical double layer. The counterions are not fixed and due to Brownian motion, colloidal particles are also not at rest. The stability of colloids can be increased by creating double layers of different materials like organic polymers over the colloidal particles. Such coated particles are called capped nanoparticles.
Synthesis of metal nanoparticles
- Synthesis of metal nanoparticles is carried out by reduction of metal salts or acid. The starting materials are called precursors.
Example (1) : Silver nanoparticles can be obtained by reducing silver nitrate with ethylene glycol (EG) in presence of polyvinyl pyrrolidone (PVP). To control the growth rate of various faces of the silver crystal, PVP is used which acts as a capping agent.
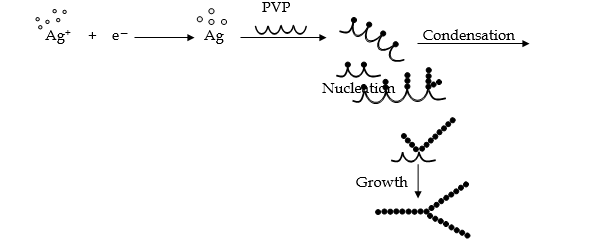
Example (2):
Gold nanoparticles are synthesized by reducing HAuCl4 (chloroauric acid) with Na3C6H5O7 ( trisodium citrate). The growth of the Au-nanoparticles is stabilized by the formation of a double-layer by oppositely charged citrate ions. Gold particles can also be stabilized by using thiol or other capping molecules. Gold nanoparticles exhibit colors like red, magenta depending upon the particle size.
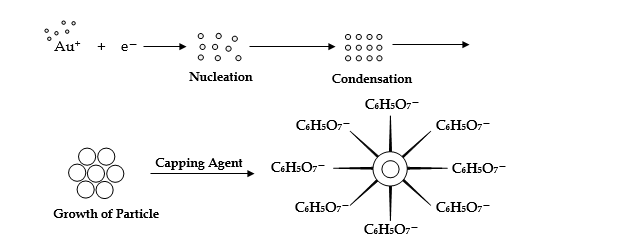
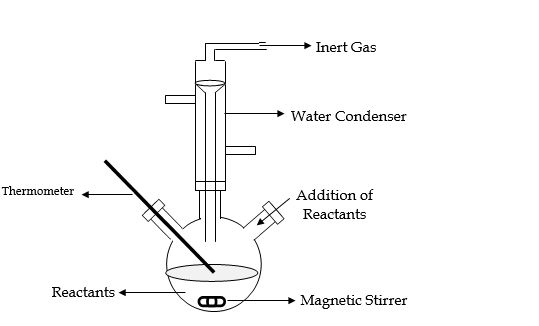
Experimental technique:
Synthesis of colloidal particles is usually carried out in a glass reactor of a suitable size. It has two openings at the sides, one to introduce reactants like precursors, gases and the second to measure temperature, pH, etc. during the reaction. Reactions are carried out in an inert atmosphere to avoid oxidation of colloidal particles, Reaction mixture can be stirred with the help of Teflon coated magnetic stirrer.
Preparation of nanoparticles by Sol-gel method
Explain the preparation of nanoparticles by the Sol-gel method
Sol-gel is a wet-chemical technique to prepare nanomaterials and is also known as the chemical solution deposition method. This method is mainly used in material science and in ceramic engineering.
Sols are colloidal solutions of solid particles suspended in a liquid. Gels are jelly-like colloidal systems in which sol particles are interlinked and form a continuous network. Hence, the gel is a colloidal system in which liquid is dispersed in a solid medium.

By drying the liquid of gel, it is possible to obtain nanomaterials in the form of powder, thin film etc. Nanorods, nanotubes, nano-particles can be prepared by this technique.
Synthesis
Synthesis of gel nanoparticles is done by carrying out the process of hydrolysis, condensation, and polycondensation of metal oxides, alkoxides, or metal chlorides. These starting materials are called precursors.
Example: tetraethyl silicate, Si(OC2H5)4
Step-I: hydrolysis
Si(OC2H5)4 + H2O → HO—Si(OC2H5)3 + C2H5—OH
Step-II Condensation reaction,
(C2H5O)3—Si—OH + HO—Si— (OC2H5)3 → (C2H5O)3Si—O—Si(O C2H5)3 + H2O
To get the desired structure, liquid filled in the pores of gel is removed. The technique used to remove excess liquid decides the texture or distribution of pores in the gel. The final structure of the product depends upon the phase separation process used for the removal of excess liquid. The liquid from the gel is extracted in such a way that network of particles is not destroyed. The methods used are (1) the Supercritical extraction method, and (2) the Subcritical drying method.
Applications of Nanomaterials
- In the field of electronics–
The size of electronic items is tremendously reduced because of nanomaterials and their special properties like single effect and quantum effect.
- Single electron transistor (SET), Magnetic Tunnel Junctions (MTJ) are fast, compact and cheaper solid state devices used in various electronic items.
- Quantum effects are used in opto-electronic devices. They provide faster switching time, larger laser power and tunneling effect. Flat panel televisions, computer monitors, mobiles are the best examples.
2. In the field of energy–
- Portable electronic items like mobile phones, navigation devices, laptop computers, remote sensors, calculators etc., requires light weight batteries or cells that are prepared using nanomaterials. For example, nickel metal hydride batteries
- Solar cells are made using nanomaterials. Hydrogen fuel is made using photocatalysts of nanomaterials.
3. In the automobiles industry:
- Nanoparticles in paint are used to give attractive thin coatings.
- Nanoparticles of TiO2 are used to develop self cleaning glass.
- Alloys of nanoparticles of Ni—Ti are being used in small motors as they perform better and use less power.
- By using nanomaterials, light weight, thin tyres using less rubber can be prepared.
4. In sports and toys
Good quality sports equipment using nanomaterials is being used. Tennis balls using nano clay are able to fill pores effectively so that they can sustain air pressure inside the ball. This increases the life of the ball.
5. In cosmetics, sunscreens and textiles
- Nanosize TiO2 and ZnO are used in some sunscreens, as they absorb and reflect ultraviolet rays, hence they protect the skin.
- Nano particles are also used in face cream, hair cream or gel.
- Some clothes prepared by nanotechnology using special threads and dyes gives pleasant look of synthetic fibre but comfort of cotton.
6. Domestic use
- Silver nanoparticles are used in air purifiers and water purifiers. They also show antibacterial properties.
- Nanomaterials are used for making decorative glasses, window materials used in houses.
7. In biotechnology and medical field:
- Antibacterial Ag-coating on wound dressing
- Steel with titanium oxide nano particles can be used in implantation for orthopaedic and heart values.
- Magnetic materials made from nano material are used in analytical instrument like magnetic resonance imaging (MRI) used in hospitals.
- They are used for detection of tumors and for treating them by drug delivery.
- Nano technologies are used to detect viruses, E.Coli, DNA, proteins. It is going to help and cure diabetic and HIV affected patients.
8. In defense and space
- Nanomaterials can be used in space-craft, and defence equipment because of their light weight.
- Aerogels are poor conductors of heat and can be used to prepare high temperature materials. They can be used as sensor to detect biological weapons.
- Polymer based nano composites are better protectors for radiations.
Ostwald ripening
As the concentration of reactants in the solution increases, the formation of nuclei starts at a certain concentration say Co. Further rise in concentration, increases nuclei formation up to concentration say CN, above which supersaturation takes place. After concentration, CN, crystal growth starts and it reduces the concentration of solute till equilibrium is established. It is important to control the concentration of solute so that no fresh nuclei are formed, once the concentration CN is reached so that particles grow properly in size. Size of particles increases at the cost of small particles present in the solution. Larger particles are most stable because as they grow, their surface-free energy is reduced. This process of growth of the particles is known as Ostwald ripening.
FAQs
What is Nano science?
Nanoscience can be defined as the study phenomena and manipulation of materials at atomic, molecular, and macromolecular scales where properties differ significantly from those at a large scale.
What is Nano technology?
Nanoscience can be defined as the design, characterization, production, and application of structure and devices by controlling shape and size at the nanometer scale.
Give advantages of Chemical Methods of preparation of Nanomaterials.
Nanomaterials are prepared below 623 K.
They involve less instrumentation. Therefore are less expensive and are simple.
Nanomaterials are obtained in the form of liquid which can be easily converted into dry powder or thin film etc.
Nanoparticles of different sizes or shapes are possible.
Foreign ions can be introduced during synthesis depending upon the requirement of the materials.
Quality articles is the main to invite the visitors to pay a visit the site, that’s what this site is providing.
Today, while I was at work, my cousin stole my iPad and tested to see if it can survive a forty foot drop, just so she can be a youtube sensation. My iPad is now broken and she has 83 views. I know this is completely off topic but I had to share it with someone!
Definitely believe that which you stated. Your favorite reason seemed to be on the net the easiest thing to be aware of. I say to you, I certainly get annoyed while people consider worries that they plainly do not know about. You managed to hit the nail upon the top as well as defined out the whole thing without having side-effects , people could take a signal. Will probably be back to get more. Thanks
Thank you very much.
You are a very intelligent person!
Ohh…. very nice. Thank you Saul and love you. Have a great day
Heya i’m for the first time here. I found this board and I find It really useful & it helped me out much. I hope to give something back and help others like you aided me.
Thank you Tuan
I believe you have remarked some very interesting details, thankyou for the post.
Thank you Nicolas
Hey there, i just now thought id post and tell you your weblogs structure. It looks brilliant on the Google Chrome internet browser. Anyhow maintain the good work.