Introduction
Electrical Conductors such as metals contain a large number of free electrons which can move throughout the crystal lattice. A directional flow of these electrons constitutes an electric current. In Normal conditions, these moving electrons continuously suffer collisions with intervening atoms and the crystal lattice, due to which their motion is opposed. This opposition to the flow of electrons is called the resistance of the material of the conductor which is characteristic of materials. Due to these collisions, the flowing electrons continuously lose energy which has to be supplied by a source of emf in order to maintain the current. So the applied voltage must be maintained without a break in order to keep the current going.
However, in superconductivity, the conducting electrons move through the crystal lattice without losing energy as a result of cooper pairs. Consequently, a current once started in a superconductor will keep on flowing even without a voltage source as there is no way for the electrons to lose their energy.
Critical temperature [Tc.]
Superconductivity was discovered by Dutch physicist Heike Kamerlingh Onnes. He studied the variation of electrical resistance with temperature, particularly at very low temperatures. He selected Hg for his studies because it can be easily obtained in a pure state by distillation. Initially, as the temperature was gradually lowered, there was a fall in resistance because the kinetic energy of the crystal lattice decreases. At a temperature of about 4.2 K, the electrical resistance of the frozen mercury sample suddenly disappeared, leading to superconductivity. This temperature is called Transition Temperature or Critical Temperature [TC].
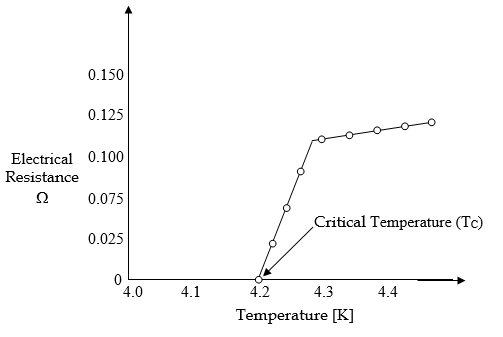
A material will display superconductivity only below critical temperature or Transition Temperature. At transition temperature or below, the crystal lattice has little thermal motion and the electrons move through the crystal lattice without losing energy as a result of cooper pair formation. Therefore, a current once started in superconductivity, keeps circulating forever, without the need for an external source of emf.
Meissner effect
When a normal conductor is placed in a uniform magnetic field, the magnetic lines of force will penetrate through it. The extent to which the lines pass through is called Magnetic permeability (m). This depends on the nature of the substance. Meissner found that when a superconductor is placed in a weak magnetic field, it expels all the magnetic flux or lines of force. This complete exclusion of the applied magnetic field is known as the Meissner effect. In superconductors, the magnetic permeability is almost Zero. Thus it behaves as a perfect diamagnetic substance.
A magnetic field has energy and when a superconductor is placed in a magnetic field, it becomes energetic. To acquire a lower energy state, the superconductor sets up a current in its surface layer, these currents in turn set up magnetic fields which cancel the external applied magnetic field.

Conventional Superconductors
Superconductors that require liquid He for cooling are called conventional superconductors.
Nb shows the highest critical temperature of 9.4 K. Hence a large number of compounds and alloys of Niobium have been studied like Nb3Sn, Nb3Ge, Nb3Al, and NbN. Among these Nb3Ge posses the highest critical temperature of 23.3 K.
Niobium alloys are brittle and cannot be fabricated in the form of wires. So far V3Ga and Nb3Al have been manufactured to use as a coil. However, preparations of conventional superconductors are difficult and their mechanical properties are poor.
Organic Superconductor
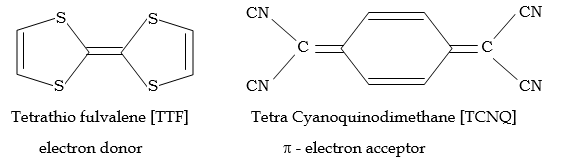
Most organic and organometallic substances are insulators. Organic superconductors are charge transfer complexes formed between the pi-electron donor and pi-electron acceptor. It shows excellent conductivity at 85K. Eg. TTF – TCNQ TTF = Tetra thiofulvalene TCNQ = Tetra Cyano quinodimethane
At present two classes of organic superconductors are known.
- Behgaard salt: Superconductivity is also found in a Selenium-based organic compound called Behgaard Salts. These also charge transfer salts of the type
(TMTSF)2 X. Where TMTSF = Tetramethyl tetraselenofulvalene
X = PF6—, AsF6—, NbF6— and ClO4—

- (BEDT–TTF)2X family: There is another class of Organic superconductor with the composition (BEDT – TTF)2X. Where BEDT – TTF = bis (ethylene dithio) tetra thio fulvalene.
X = I—, PF6—, AsF6— and ClO4—
Fullerenes & alkali metal fullerides.
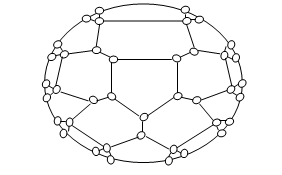
- Fullerene was discovered by H.W. Kroto.
- It was prepared by Vapourization of graphite using a laser.
- It is a C60 Molecule. Each carbon atom is sp2 hybridized.
- It possesses 20 six-membered rings and 12 five-membered rings.
- The presence of a 5-membered ring makes C60 molecule spherical in structure.
- It is named Buckminster fullerene after the famous architect of Geodesic domes R. Buckminster fuller.
- It possesses FCC structure with a lattice constant of 14.2 A0.
- Since the radius is 3.54 A0, there exist large vacancies to accommodate dopants of small radii. C60 molecule possesses three voids. One is the octahedral void and the other two are tetrahedral voids. When these three voids are filled with alkali metal atoms, the superconducting MxC60 is formed. In MxC60, M is K or Rb and x varies from 1 to 6. Superconductivity is highest when x = 3.
| Sr. No. | MxC60 | Tc |
| 1. | K3C60 | 18 K |
| 2. | Rb3C60 | 30 K |
| 3. | Rb2.7 Tl2.2 C60 | 45 K |
High-Temperature Superconductors
- Chu and Wu discovered a 93 K high-temperature superconductor, Y Ba2 Cu3 O 7 – x. This is called as 1 – 2 – 3 type of compound because of the ratio of the metal present.
- High-temperature superconducting material is prepared by mixing various oxides or carbonates in suitable proportions followed by heat treatment.
- The structure of this compound is a modified Perovskite structure. The three cubic perovskite units are stacked one on top of the other giving an elongated (tetragonal) unit cell.
- The upper and lower cubes have a Ba2+ ion at the body-centered position and the smaller Cu2+ ions at each corner. The middle cube is similar but has Y3+ ion at the body center. The stoichiometry of this compound would be YBa2Cu3O9 but actually, its formula was found to be YBa2Cu3O 7– x. This is because there is a massive oxygen deficiency and about one-quarter of the oxygen sites in the crystal are vacant.

Applications of superconductors.
1) Alloys of Nb3Sn and Nb3Ge are used to make the wires for extremely powerful electromagnets which are used as,
a. In linear accelerators, they are used as atom smashers for high-energy particles.
b. They are used in nuclear fusion research to make powerful magnetic fields that act as magnetic bottles for plasma.
c. They are used in chemical laboratories for nuclear magnetic resonance machines.
d. They are used to produce extremely high magnetic fields of about 19 T. The superconducting quantum interference devices called SQUID are used to detect tiny variations of magnetic flux density ~10-5 T.
e. SQUID can also be used in electronics to make ultra low noise amplifiers.
2. Potential uses
a. Low loss transmission of DC through resistance-less cables from the electricity power station.
b. In computers if superconductors are used then heat problems would be reduced.
c. In Japan a prototype of a train has been built up which floats on a magnetic field based on a superconducting phenomenon.
FAQs
What are examples of superconductors?
Niobium, magnesium diboride, cuprates such as yttrium barium copper oxide, and iron pnictides. These materials only become superconducting at temperatures below a certain value, known as the critical temperature. Some organic superconductors include fullerenes and carbon nanotubes.
What are superconductors used for?
There are several uses, Kindly refer to the applications part in the above article.

I just could not depart your web site before suggesting that I extremely enjoyed the standard information an individual supply to your visitors? Is gonna be again ceaselessly to check up on new posts
Thank you dear
Hey! That’s a extremely great post. I’m very certain I will suggest it to my co-workers.If you post extra posts please e-mail them to me.
Thank you very much dear..
I¦ve recently started a website, the information you provide on this web site has helped me greatly. Thanks for all of your time & work.
Thank you Dorian
I besides conceive so , perfectly composed post! .
Its superb as your other blog posts : D, thanks for putting up. “The squeaking wheel doesn’t always get the grease. Sometimes it gets replaced.” by Vic Gold.
Thx
I cling on to listening to the newscast lecture about receiving free online grant applications so I have been looking around for the top site to get one. Could you tell me please, where could i get some?
Have you modified this theme yourself? Or is it a premium paid theme?
It is free theme..