What is Pinacol – Pinacolone Rearrangement | Pinacol – Pinacolone Rearrangement kya hai?
Molecular Rearrangement reaction & Types of rearrangement reactions:
The reactions involving the migration of an atom ( like H) or group (like methyl, ethyl, etc) from one atom to another (i.e. change in the bonding sequence within a molecule) are known as molecular rearrangements or rearrangement reactions.
Rearrangements are divided into two types, intermolecular and intramolecular rearrangement reactions. Pinacol – Pinacolone rearrangement is an example of an intramolecular rearrangement reaction.
In the intramolecular process, the group that migrates is not completely detached from the system in which rearrangement is taking place. In contrast, in the intermolecular process, the migrating group is first detached and later re-attached at another site.
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged with a carbocation intermediate to give a structural isomer of the original molecule.
Explain the Principle of Pinacol – Pinacolone Rearrangement with a mechanism and one application.
Principle of Pinacol Pinacolone Rearrangement
Acid-catalyzed conversion of vicinal diol, 1, 2 – diol, or vicinal glycol to give highly substituted ketone is known as Pinacol – Pinacolone rearrangement.
The pinacol rearrangement is the acid-catalyzed dehydration of glycols, which converts the glycol into an aldehyde or a ketone.
It is one of the simplest systems where an alkyl group migrates, with its bonding pair, to an electron-deficient carbon atom.
General Reaction

Example

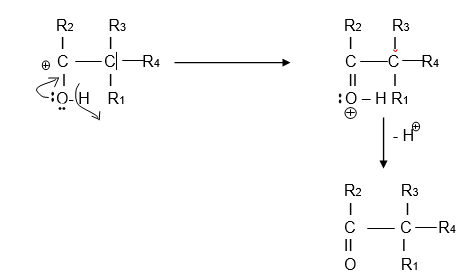
Mechanism of Pinacol Pinacolone Rearrangement
Pinacol Pinacolone Rearrangement takes place via four steps,
Step I:- Protonation
In step-I protonation take place with the help of acid. The catalyst is either mineral acids (dilute H2SO4 or H3PO4) or Lewis acids (AlCl3, BF3, and ZnCl2) or any electrophilic reagent (PCl5). Proton gets attached to either of the oxygen atoms.

Step II:- Formation of carbocation
In this step, the water molecule eliminates with the formation of a carbocation. Tertiary carbocation formation takes place.

Step III – Rearrangement of the carbocation.
It is an important step in this reaction. Intramolecular rearrangement takes place between two adjacent carbon atoms with a shifting of the alkyl or aryl group. It is also called as 1-2 shift. Carbocation shifting to the adjacent position is also observed in this step.

If the glycol is unsymmetrically substituted then there is a choice (regioselective choice) of preferential protonation in the hydroxyl group. Of the two hydroxyl groups, the one that forms the more stable carbenium ion is protonated preferentially.
The migrating groups depend on various factors like, electron-donating & electron withdrawing factors, steric hindrance, and other strain factors on the rearrangement during migration.
Stereoelectronic factors and conformational effects also play an important role in determining whether a particular migration is favoured.
The reaction conditions (i.e. type of acid, concentration, solvent, and temperature) also make influence the course of the rearrangement reaction.
The usual migration was observed generally as Aryl>H > alkyl. But the migration of H is often unpredictable. In some cases, migration of hydrogen is preferred to that of aryl and in other cases, migration of alkyl is preferred to that of H.
Step IV:- Deprotonation
In the final step, deprotonation leads to the formation of stable pinacolone.
Applications of Pinacol Pinacolone Rearrangement.
Pinacol Pinacolone rearrangement is a very important process in organic chemistry.
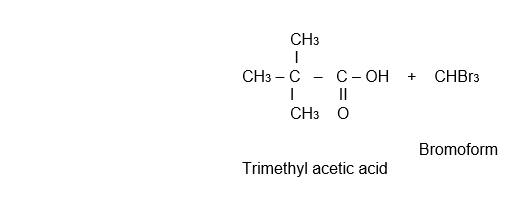
Tetramethyl ethylene glycol is heated with H2SO4 giving methyl t- butyl ketone. This haloform reaction gives trimethyl acetic acid.

Pinacolone is used in manufacturing Pesticides, Fungicides, and Herbicides.
It plays an important role as an intermediate for biologically active compounds like antiviral, antibacterial, antifungal, and antituberculous drugs. Pinacolone is also used to prepare the cyanoguanidine drug – pinacidil. Another use of Pinacolone is its use in Stiripentol, which is used in the treatment of epilepsy.
Pinacolone is also used to produce triadimefon which is used in the agriculture industry. It is also used to prepare pinacidil, as well as naminidil.
FAQs
What is pinacol Pinacol Pinacolone rearrangement?
Acid-catalyzed conversion of vicinal diol, 1, 2 – diol, or vicinal glycol to give highly substituted ketone is known as Pinacol – Pinacolone rearrangement
What is the product of the Pinacol Pinacolone rearrangement reaction?
Highly substituted ketone which is also known as Pinacolone.
Application of pinacol pinacolone rearrangement?
Pinacolone is used in Pesticides, Fungicides, and Herbicides.
What is vicinal diol?
Vicinal diol is an organic compound in which the two hydroxy functional groups are attached to adjacent carbon atoms. Examples include 1,2-ethanediol or ethylene glycol.
What is geminal diol?
Geminal diol is an organic compound in which two hydroxy functional groups are attached to the same carbon atom. Generally, organic geminal diols readily dehydrate to form a carbonyl group
What is a Rearrangement reaction?
A rearrangement reaction is an organic reaction in which an atom, ion, group of atoms or chemical unit migrates from one carbon atom to another carbon atom in the same or different molecule resulting in a structural isomer of the original molecule. The reaction often includes the breaking and/or making of carbon-carbon sigma bonds.
Refer – Beckmann Rearrangement, Hofmann Rearrangement, and Benzilic acid Rearrangement to understand the concept of rearrangement reaction.




Wow, fantastic weblog format! How lengthy have you been blogging for? you make running a blog glance easy. The overall look of your web site is excellent, let alone the content!
Good post right here. One thing I would really like to say is always that most professional fields consider the Bachelors Degree like thejust like the entry level standard for an online certification. Even though Associate College diplomas are a great way to begin, completing your own Bachelors reveals many good opportunities to various professions, there are numerous online Bachelor Course Programs available by institutions like The University of Phoenix, Intercontinental University Online and Kaplan. Another concern is that many brick and mortar institutions provide Online types of their qualifications but typically for a drastically higher charge than the providers that specialize in online college degree plans.
Thanks for your article. I have usually observed that most people are eager to lose weight when they wish to appear slim plus attractive. Nevertheless, they do not usually realize that there are other benefits just for losing weight also. Doctors assert that over weight people are afflicted with a variety of health conditions that can be instantly attributed to their particular excess weight. The good thing is that people who sadly are overweight and also suffering from diverse diseases can help to eliminate the severity of their own illnesses through losing weight. It’s possible to see a continuous but noticeable improvement with health when even a small amount of fat loss is realized.
I appreciate, cause I found just what I was looking for. You have ended my four day long hunt! God Bless you man. Have a nice day. Bye
Great article.Much thanks again. Keep writing.
Very nice post. I just stumbled upon your weblog and wished to say that
I’ve truly enjoyed browsing your blog posts. In any case I’ll be subscribing to your rss
feed and I hope you write again very soon!
Terrific work! This is the type of info that should be shared around the net. Shame on Google for not positioning this post higher! Come on over and visit my website . Thanks =)
Thank you
WONDERFUL Post.thanks for share..more wait .. …
Thank you
I’m not sure why but this web site is loading very slow for me. Is anyone else having this issue or is it a issue on my end? I’ll check back later on and see if the problem still exists.
Hello! I could have sworn I’ve been to this site before but after reading through some of the post I realized it’s new to me. Nonetheless, I’m definitely happy I found it and I’ll be bookmarking and checking back often!
I just couldn’t go away your website prior to suggesting that I really enjoyed the standard info an individual provide on your guests? Is going to be again ceaselessly to investigate cross-check new posts.
whoah this blog is magnificent i love reading your posts. Keep up the good work! You know, many people are hunting around for this info, you could help them greatly.