Molecular fluorescence and phosphorescence spectroscopy | Fluorimetry and Phosphorimetry – instrumentation, and applications |
An excited molecule comes back to the ground state by emitting an absorbed light in the form of UV light or visible light photon. This process is called Fluorescence.
Fluorimetry and Phosphorimetry

The study of fluorescence is called Fluorimetry and that of phosphorescence is called Phosphorimetry
The molecule has different electronic energy levels. Each electronic energy level is subdivided into different vibrational energy levels. Let E0 be the ground state of the hypothetical molecule. It is subdivided into vibrational energy levels denoted by 0,1,2 . . . . The vibrational energy level no. 0 has minimum energy in the ground state. Let E1 be the excited state which is subdivided into vibrational energy levels denoted by 0,1,2…. The vibrational energy level no. 0 has minimum energy in an excited state.
Initially, the molecule is present in the ground state and present in vibrational energy level no.0 as it has minimum energy.
When UV or visible light is incident on the molecule, it absorbs a photon within 10-15 seconds and gets excited to any one of the vibrational energy levels of the excited state. From an excited state, one of these possibilities can occur, depending on the molecule and condition.
a) Molecule may return to ground state without emitting light radiation due to loss of excitation energy in the form of collision.
b) Molecule may come back to the ground state by emitting a UV light or visible light photon. This process is called Fluorescence. The emission occurs within 10-6 to 10-4 sec of absorption of light. The fluorescence spectrum is shifted to a longer wavelength compared to the wavelength absorbed; this is because of loss of energy in the form of vibration in the excited singlet state since vibration requires 10-13 sec which is less than the decay time of fluorescence. (If Source radiation is removed Fluorescence stops hence it is called an Instantaneous Phenomenon)c) The third possibility is that a molecule with a relatively stable excited singlet state may undergo a transition to a metastable Triplet state {i.e. undergo Intersystem crossing (I.S.C)} and from there, it comes back to the ground state emitting U.V light or visible light photon. This process is called Phosphorescence. The emission occurs within 10-4 to a few seconds of absorption of light. The decay from singlet to triplet is forbidden by spin symmetry and hence is slow. Thus Phosphorescence lifetime is longer than Fluorescence. Since the triplet has less energy than the singlet, it lies lower, as per Hund’s rule and hence Phosphorescence radiation has still a longer wavelength than both the absorbed as well as Fluorescence. (If Source radiation is removed Phosphorescence continues for a few seconds hence it is called a Delayed Phenomenon, which can be seen in the dark)
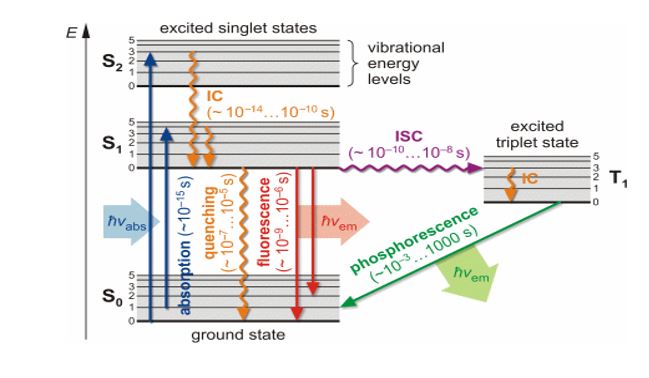
The electrons present in the ground state of the molecule are paired i.e. they have opposite spins. When the molecule is excited to a higher energy level, one of the electrons of the ground state is excited to the higher energy level but still, it has the opposite spin as compared to that of the ground state. This excited state in which the electron has the opposite spin is called an excited singlet state.
When the molecule returns from the excited singlet state to the ground state, the two electrons get paired again. The excited singlet state has more energy and thus the electron immediately returns to the ground state and the process is called fluorescence.
When the molecule is excited to a higher energy level, one of the electrons of the ground state is excited to the higher energy level but it has the same spine as compared to that of the ground state. This excited state in which the electron has the same spin is called an excited triplet state. This transition is less probable.
The excited singlet state has less energy and thus the electron remains in the excited state for some time and comes back to the ground state. Thus the emission is delayed. This process is called is phosphorescence.
The study of fluorescence is called Fluorimetry and that of phosphorescence is called Phosphorimetry. Fluorescence ceases when the source of light is removed while phosphorescence even after the source of light is removed.
The difference, between fluorescence Vs Phosphorescence
| No. | Fluorescence | Phosphorescence |
| ] | Instantaneous emission of absorbed light. | Delayed emission of absorbed light. |
| 2 | The time interval between absorption and emission of light energy is 10-6 to 10-4 sec | The time interval between absorption and emission of light energy from 10-4 to a few sec |
| 3 | The transition of an electron from excited singlet to ground state. | The transition of an electron from excited triplet state to ground state. |
| 4 | Phosphorescence continues in the absence of source radiation for a few seconds. | Phosphorescence continues in absence of source radiation for a few seconds. |
| 5 | Instrumentation is less complicated. | Instrumentation is more complicated. |
| 6 | Less sensitive. | More sensitive. |
The mathematical expression between fluorescence intensity and concentration of a given solution
The relation between the intensity of fluorescence F, and the concentration of the
Fluorescence substance C can be established as follows,
F α Io-It
F=K(Io-It) ——— (I)
Where, F=intensity of fluorescence radiation
K=proportionality constant
It=Intensity of transmitted light
Io= Intensity of incident light
According to Beer-Lambert’s law,
Log10 Io / It = εbc
Log10 It /Io = – εbc
Therefore It = Io .10 – εbc
It = Io . e-2.303 έbc ————(II)
Substituting eq.(II) in eq.(I) we get,
F=K(Io– Io . e-2.303)
F=K Io(1- e-2.303 έbc)
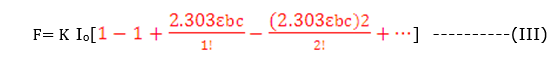
For a dilute solution, εbc is very small and εbc < 0.05 ,eq. (III) reduces to
F= K Io2.303 εbc
F= K’C
K’=K Io 2.303εb
F α C ———–(III)
From the above equation, it follows that fluorescent intensity is practically proportional to the concentration of the fluorescent substances.
At higher concentrations, the fluorescence intensity may decrease with increasing concentration.
Factors affecting fluorescence and Phosphorescence
1) There is a π to π * transition in the above processes. Hence molecules with a conjugated system of double bonds or functional groups containing nonbonding or lone pairs of electrons such as – OH, -NH2 show fluorescence and phosphorescence.
2) Electron withdrawing groups such as COOH. – NO2, halogens decrease fluorescence and phosphorescence.
3) The pH of the solution affects the processes because ionization decides whether the molecule will show fluorescence or not e.g. phenol does not exhibit fluorescence but phenoxide ion shows fluorescence.
Fluorimeter
What is Fluorimeter? or Fluorimetry principle
It is the instrument for measuring the intensity of fluorescent light.
Schematic diagram of Fluorimeter

Fluorometry instrumentation
- Source: Hg vapor lamp, which produces UV light.
- Convex lens: To produce a parallel beam.
- Primary filter: used to select wavelength which is able to excite sample to show fluorescence.
- Sample cell: Made up of quartz..
- Secondary filter: It absorbs the primary radiant from the source light but allows the fluorescent light emitted by the sample to pass and fall onto the photocell.
- Photocell: Converts the emitted fluorescent light radiation into an electrical signal.
- Recorder / Readout: Displays the reading in digital or analog form.
Working of Fluorimeter
Let’s understand the working of a Fluorimeter. First, the cell is filled with blank or distilled water, and the fluorescent intensity is set to read zero. A series of standard solutions are prepared. The blank solution is then replaced by each standard solution in the decreasing order of concentrations and the fluorescence of each solution is measured. The fluorescence of the unknown solution is measured. The calibration curve of fluorescent intensity against concentration is plotted and the concentration of unknown is determined.
Applications of Fluorimetry
Following are the applications of Fluorimetry
1) In the detection of metal ions: Certain metal ions form fluorescent complexes with organic reagents. They can be detected by this method. e.g. Al, Be, Zn with 8-hydroxy quinoline, ruthenium with 5-methyl-1, 10-phenanthroline, and uranium with NaF.
2) Quantitative analysis: Boron forms a fluorescent complex with benzoin. This property can be used in its quantitative estimation.
3) Fluorescent indicators: The intensity and colour of certain fluorescent substances such as eosin depend on pH. They can be used in acid-base titration, the titration of coloured solutions.
4) Fluorometric reagents: Certain aromatic reagents like benzoin from fluorescent chelates with metal ions like zinc.
5) Organic and biochemical analysis: Estimation of vitamin B1, steroids, and enzymes forms fluorescent products which can be estimated from food products, pharmaceuticals, and clinical products.
Phosphorimeter
What is Phosphorimeter?
The molecules, that show fluorescence, also show phosphorescence. Thus for the determination of phosphorescence, after the incident light is incident on the sample, the source of light is removed or the source of light is cut off from the sample.
Schematic diagram of Phosphorimeter
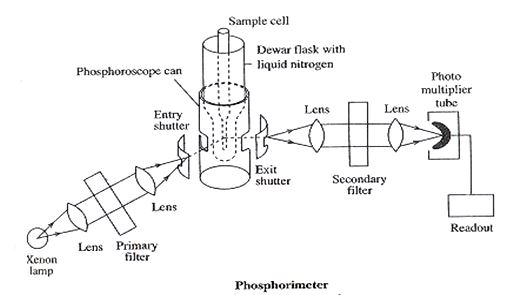
- Source: Xenon lamp, which produces UV light.
- Convex lens: To produce a parallel beam.
- Primary filter: used to select wavelength which is able to excite sample to show phosphorescence.
- Sample cell: The UV light then passes through the entry slit, which can be closed by the shutter. The UV light then passes through the phosphoroscope. The phosphoroscope contains a Dewar flask, which is kept at the lower end and contains liquid nitrogen, the temperature of liquid nitrogen is – 1960 C. The sample is placed in a quartz tube in the sample tube, which is placed in a Dewar flask. At low temp collisions with other molecules are avoided. After irradiation, the light is cut off by rotating the phosphorescence can.(since in the presence of light radiation fluorescence is also shown to a less extent and hence needs to be removed) Now sample only shows phosphorescence and the emerging phosphorescent light passes through the exit slit to sec filter.
- Secondary filter: It absorbs the primary radiant light from the source but allows the phosphorescent light emitted by the sample to pass and fall on the photocell.
- Photocell: Converts the emitted phosphorescent light radiation into an electrical signal
- Recorder / Readout: Displays the reading in digital or analog form
Working of Phosphorimeter
A series of standard solutions of known concentration of the substance is prepared in an organic solvent. Initially blank is introduced in the sample cell and fluorescent intensity is adjusted to read zero. The solution of the highest concentration is introduced into the cell and its fluorescent intensity is adjusted to 100% on the instrument. The fluorescent intensities of different solutions are recorded. Similarly, the unknown solution is introduced into the cell and its fluorescent intensity is recorded.
The graph of fluorescent intensity against concentration is plotted, from which the concentration of unknown can be calculated.
Applications of Phosphorimetery
- Determination of aspirin from blood serum. Aspirin is phosphorescent and its metabolic product, salicylic acid is fluorescent.
- Determination of cocaine, procaine.. etc from blood serum and urine.
- Determination of alkaloids such as nicotine from crude tobacco.
- In environmental studies.
FAQs
What is meant by Fluorimetry and What is meant by Phosphorimetry?
The study of fluorescence is called Fluorimetry and that of phosphorescence is called Phosphorimetry
What is the principle of fluorimetry?
When the molecule returns from the excited singlet state to the ground state, the two electrons get paired again. The excited singlet state has more energy and thus the electron immediately returns to the ground state the process is called fluorescence.
What is a fluorometer used for?
It measures the fluorescence or light emitted by different fluorescing objects
I truly appreciate this post. I抳e been looking all over for this! Thank goodness I found it on Bing. You’ve made my day! Thx again
Thank you dear
Hi, just required you to know I he added your site to my Google bookmarks due to your layout. But seriously, I believe your internet site has 1 in the freshest theme I??ve came across. It extremely helps make reading your blog significantly easier.
Thank you very much dear…..
Hey very nice blog!! Man .. Excellent .. Superb .. I’ll bookmark your website and take the feeds also?KI’m glad to seek out a lot of useful info right here within the put up, we need work out extra techniques in this regard, thanks for sharing. . . . . .
Thank you very much
Im no longer sure the place you are getting your information, but great topic. I must spend a while learning much more or figuring out more. Thank you for great info I used to be looking for this info for my mission.
Thank you
Greetings! Very helpful advice on this article! It is the little changes that make the biggest changes. Thanks a lot for sharing!
Thanks for your feedback.
Somebody essentially help to make seriously articles I would state. This is the first time I frequented your website page and thus far? I surprised with the research you made to create this particular publish incredible. Fantastic job!