Thermogravimetric Analysis TGA principle, instrumentation | Application of thermogravimetric analysis
Thermal Analysis is a method in which physical or chemical properties of a sample (pure substance or mixture of substance and/or reaction mixture) are measured as a function of temperature or time, by the sample is subjected to a control temperature program.
Thermogravimetric Analysis Principle (TGA)
In thermogravimetry, the change in the mass is measured with respect to temperature or time. There is a change in mass when volatile substances are formed. The volatile substance includes water of crystallization which gets evaporated. These changes in mass when the sample undergoes a decomposition reaction.
In addition to decomposition, there can be oxidation depending on the surrounding atmosphere. The measurement of mass is made by using a thermal balance and is a step in the graph thus is plotted to a particular reaction and substance.
In principle, only that process which on heating undergoes mass change can be studied by thermogravimetry
Reactant —————-> Product + Gas
OR
Reactant + Gas ———–> Product
A plot of the mass of the sample against temperature/time known as a thermograph is given below.
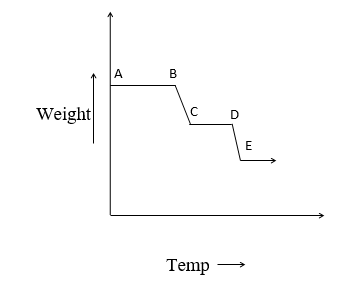
1. The horizontal region represents the region where there is no change in a mass on heating eg. AB, CD, EF.
2. The curved portion indicates weight loss. They indicate the occurrence of chemical reaction or transformation of sample eg. BC and DE region.
From the thermograph, the temperature can be determined. The temperature range is selected in thermogravimetry from room temperature of about 15000C with inert atm or reactive atm. These measurements are made in thermobalance.
Thermogravimetric Analysis Instrumentation (TGA instrumentation) – Thermobalance
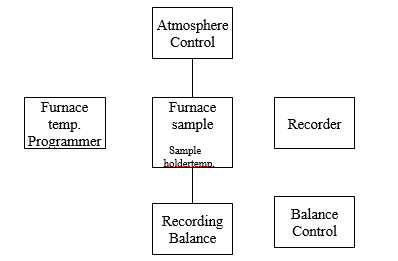
The components of thermogravimetric instrumentation include
1. A sensitive analytical balance.
2. A furnace
3. A unit for measurement and control of temperature.
4. A recorder that gives a graph of the mass of the sample as a function of temperature.
5. Equipment to control the atmosphere of the sample.
Balance
In TGA, the mass of the sample is recorded over a wide range of temperatures (15000C). The balance must satisfy the following condition.
1. It is a rapid and uniform response to the weight change over an entire temperature range.
2. The mass change should be converted to an electrical signal for continuous recording.
3. It is sensitive to medium as well as small size samples i.e. 2gm to 100gm.
4. It is precise, accurate, and reproducible, under extreme temperature and atmospheric conditions.
5. High degree of mechanical and electronic stability and it is simple to operate and versatile.
The most commonly used balance system in thermogravimetry is the null point balance in the null point system, where there is a change in weight, the balance beam deviates from the usual position. The sensor detects the deviation and initiates a force that will restore the balance to the original position. This restoring force is proportional to the change in the weight of the sample.
Furnace
The furnace should be providing a temperature range from room temperature to 15000C. The heating rate should be uniform and reproducible. Therefore controlling the device is necessary. The heating rate varies from 0.50C per min to 2.50C per min. The furnace, such that the heat of the furnace is localized over the sample, and the sample must be the same amount of heat energy so that the temperature remains uniform over the entire sample.
The sample holder is made up of quartz or alumina or steel depending upon the nature of the sample, the weight of the sample, temperature, etc. The sample holder can be a deep crucible or cup or pan. Heating can be done by using resistance heat, T.R. or microwave radiation, or heat transfer from hot liquid or gases. It should be cooled to avoid heat flow to surroundings or balance. The furnace is located as far as possible from the balance.
Temperature measurement
Temperature sensing devices are usually thermocouples placed as nearer as possible to the sample. The emf generated by the thermocouple is applied to the x-axis of the recorder.
Recorder
The change in the mass of the sample is measured in terms of electric signals that are obtained from the balance and applied to the Y-axis of the recorder and the temperature of the sample is measured from the e.m.f. generated by the thermocouple. The signals are amplified and fed to the recorder.
Atmosphere control
The composition of the atmosphere surrounding the sample is important and is necessary to monitor its properties. The atmosphere can be static or dynamic and desired composition. Thermogravimetric determination can be done in Vaccum also.
Factors affecting the thermogravimetric result
I. Instrumental factors
II. Sample characteristics
Instrumental factors:-
A] Heating rate:-
When a substance or a sample is heated at a fast heating rate, the temperature of decomposition will be higher than that obtained at a slower rate of heating.
Consider the effect of heat on single step reaction as given below
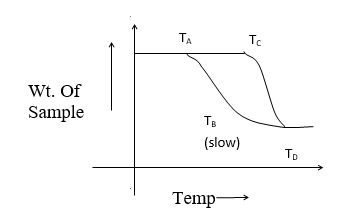
The curve AB represents the decomposition curve at a slow heating rate whereas the curve CD represents decomposition at a fast heating rate. Let TA and TC be the decomposition temp. At the start reaction and TB and TD be the final temperature on completion of decomposition.
TA< TC, TB<TD
∴TB – TA< TD – TC
Hence, if an intermediate compound is determined by thermogravimetry higher heating rate must be avoided.
B] Furnace atmosphere
The decomposition temperature of a substance depends upon the composition of the atmosphere immediately surrounding reacting substance.
Eg. the Decomposition of CaCO3 at a much higher temperature if CO2 rather than N2 is employed in the surrounding atmosphere. There are three atmospheres used in thermogravimetry.
i) Static air:-
Air from the surroundings flows through the furnace.
ii) Dynamic air:-
Compressed air from the cylinder is passed through the furnace at a major flow rate.
iii) Nitrogen gas:-
N2 gas from O2 is provided to maintain an inert atmosphere.
C] Crucible (Sample holder) Geometry
The geometry of the crucible (Sample holder) can change the slope of the T.G. curve. Crucible range from flat plates to deep crucibles of various capacities. The material used in their construction may vary from glass, alumina, metallic alloy, etc. Generally, a flat plate-shaped crucible is preferred because of the easy diffusion of evolved gas.
II. Sample characteristics
A] Weight of the sample:-
If a large sample is used, there occurs a deviation from linearly as the temperature rises. This is found in case of fast exothermic reaction
B] Sample particle size:-
The different particle sizes of the sample alter the reaction rate and curve shape. With a particle size of a smaller dimension, the decomposition takes place earlier, while with a greater particle size, the decomposition takes place at a higher temperature.
C] Compactness of the sample:-
A compressed sample will decompose at a higher temperature than a loose sample.
Swelling and foaming of the sample also affect thermo gravimetric results and depend upon weight, particle size, and method of preparation of the experimental sample.
Important link – You can also read coordination chemistry in detail by clicking on the diagram.
Application of Thermogravimetric Analysis
1. The determination of purity and thermostability of primary and secondary standards used in titrimetry.
2. The determination of correct drying and ignition temp. of substance.
3. Composition of complex, mixture determine and solid-state reaction.
4. Thermal decomposition of inorganic-organic polymeric substance.
5. Corrosion of metal in the atmosphere at elevated temperature.
6. Roasting and calcining of minerals.
What is thermogravimetric analysis?
In thermogravimetry, the change in the mass is measured with respect to temperature or time.
What is the difference between TGA and DSC?
In thermogravimetry, the change in the mass is measured with respect to temperature or time but DSC measures how much energy a sample absorbs or releases during heating or cooling.
TGA full form?
Thermogravimetric analysis
What is a Thermobalance?
It is the instrument that measures the change in weight of a sample while it is being heated.

One thought on “Thermogravimetric Analysis TGA principle, instrumentation | Application of thermogravimetric analysis”