Wastewater treatment | COD Chemical oxygen demand | BOD Biological oxygen demand | Aerobic & anaerobic process |
Biological Oxygen Demand (BOD) & its significance
What is Biological oxygen demand (BOD)
1. Definition: Biological oxygen demand indicates “the amount of O2 required for biological oxidation of organic matter present in wastewater by microorganisms”. BOD is expressed in milligrams per liter of waste.
2. Under aerobic conditions, when sufficient O2 is present for the degradation of organic materials, then carbon becomes CO2, sulphur becomes sulphate, phosphorous becomes phosphates and nitrogen is converted to ammonia and nitrates. Aerobic decomposition can be shown as follows,
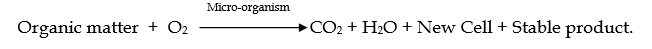
3. When the dissolved oxygen is not sufficient, carbon is converted to methane, nitrogen compounds become amines and sulphur becomes H2S. Under such anaerobic conditions, O2 may be obtained from dissolved nitrate and sulphates. Anaerobic decomposition can be shown as follows,

Significance
- BOD value is used as a measure of the degree of water pollution. Its value is proportional to the amount of organic waste present in water.
- BOD values are most useful in the evaluation of the self-purification capacity of a water body and for possible control measures of pollution.
What is five day BOD test?
The 5-day BOD or BOD5 is the amount of O2 consumed by microorganisms during the first 5 days of biodegradation. BOD5 test is performed by taking a sample of wastewater into a stoppered bottle, measuring the concentration of dissolved oxygen in the sample at the beginning of the test and again after 5 days.
To standardize the procedure, the test is run at a fixed temperature of 20oC. Since the oxygen demand of typical wastewater is several hundred milligrams per liter and since the saturated value of DO for water at 20oC is only 9.1 mg/l, it is usually necessary to dilute the sample to keep the final DO above zero. The five days BOD of a diluted sample is given by,
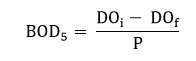
DOi = initial dissolved oxygen of the diluted wastewater.
DOf = final dissolved oxygen of diluted wastewater
P = dilution factor.
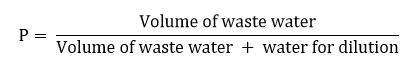
Chemical Oxygen Demand (COD)
What is chemical oxygen demand COD? How do measure chemical oxygen demand?
Chemical Oxygen Demand is “the measure of the amount of O2 required for oxidation of organic matter present in wastewater by strong chemical oxidants like K2Cr2O7 or KMnO4
COD is expressed as ppm of oxygen taken from a solution of K2Cr2O7 in 2 hours.
COD is a poor measure of the strength of organic matter as oxygen not only oxidizes organic matter but also inorganic matter such as nitrate, sulphate, reduced metal ions, and also organic materials like benzene and pyridine.
KMnO4 can oxides organic compounds which are not biologically degradable and are sometimes used to determine the COD of water. COD is a very important parameter in the management and design of treatment plants due to its rapidity in determination.
Values are taken as the basis for the calculation of the efficiency of treatment plants and also figure in the standards for discharging industrial domestic effluents in various kinds of water.
Chemical oxygen demand test – COD test
The principle involved in the determination of COD is that, when the wastewater sample is refluxed with a known excess of K2Cr2O7 in a 50% H2SO4 solution in presence of Ag2SO4 and HgSO4, the organic matter of the sample is oxidized to H2O, CO2, and NH3. The excess dichromate remaining unreacted in the solution is titrated with a standard solution of Fe(II) ammonium sulphate (FAS). The COD of the sample is calculated as follows,
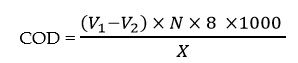
V1 = volume of FAS run down in the blank.
V2 = Volume of FAS run down in the test sample.
N = Normality of FAS
X = Volume of the sample taken for the test.
Chemical oxygen demand sensor
Nowadays photochemical sensors are also available to measure chemical oxygen demand easily.
Total Organic Carbon (TOC)
Total Organic Carbon determines the total organic matter in wastewater samples as CO2 with modern instruments.
In various TOC analyzers, a non-dispersive IR detector is used to measure the CO2 formed on combustion of the carbonaceous content. A micro liquid of the water sample is injected into a heated, packed tube through which passes a stream of oxygen or purified air. The water is vaporized and the organic content is oxidized to CO2, which is measured in an IR analyzer unit. Inorganic carbonates may interfere and these effects can be minimized by preliminary acidification of the sample.
In other TOC instruments, the carbonaceous matter is converted into methane. The organic component of a micro-sample is first pyrolyzed at 850oC in presence of CuO oxidant and is then conveyed into a tube, packed with nickel catalyst supported on activated alumina which is maintained at 300-400oC. The amount of CH4 produced is determined by means of the Flame ionization detector.
Aerobic & Anaerobic process
Wastewater can be treated by either of the following processes.
Aerobic wastewater treatment
- In the Aerobic process, biodegradation of organic matter in wastewater is done by microorganisms in presence of O2.
- Certain microorganisms, in presence of dissolved oxygen and in proper environmental conditions, utilize organic waste as their food and convert it into simpler compounds such as CO2, H2O, NO31- and SO42-, which are non-pollutants
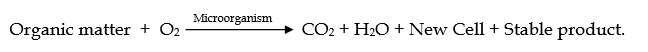
3. Among available aerobic processes, the activated sludge process is the most important process.
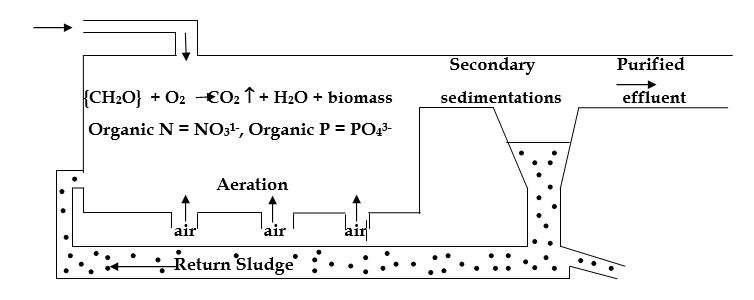
Activated sludge process
- In this process, the sewage or industrial wastewater is aerated in a reaction tank in which some microbial floc is suspended. The microorganism comes in contact with the dissolved organics and adsorbs them rapidly on their surface. The organic compounds are oxidized to CO2, H2O, and more microorganisms are produced.
- Once the food has been utilized, the microorganisms are separated from the liquid in the settling tank. It is known as a secondary clarifier. In the clarifier, they exist without food and therefore they are activated sludge. Hence the name activated sludge. The settled microorganisms are then pumped into the head of the aeration tank and the process starts all over again. If some of them are not removed, the solid mass will increase and will clog the tank. Therefore, part of the activated sludge has to be disposed of.
- By this method, 90-95% of BOD is removed.
Anaerobic wastewater treatment i.e. anaerobic treatment of wastewater
- In the Anaerobic process, biodegradation of Organic Matter in wastewater is done by micro-organisms in absence of O2
- Certain microorganisms in absence of oxygen, utilize organic waste as their food and convert it into simpler compounds such as CH4, NH3, and H2S which are pollutants.
- Under anaerobic conditions, certain groups of microorganisms like hydrolyze and methane forming organisms can carry out the digestion of complex organic wastes
- In anaerobic processes, about 95% of biodegradable carbon is decomposed into biogas and the rest 5%into biomass.
- Three steps are mainly involved in the breakdown of organic waste under anaerobic conditions. These steps involve hydrolysis of biopolymers to monomers, fermentation of monomers to volatile acids, and methanogenesis
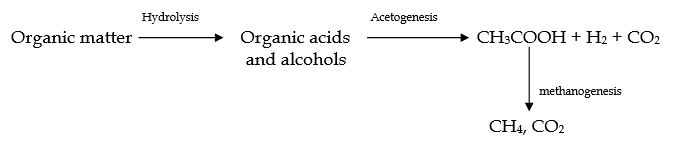
6. In the anaerobic degradation of carbohydrates, the first polysaccharide is converted to pyruvate and then pyruvate to lactic acid or ethanol.
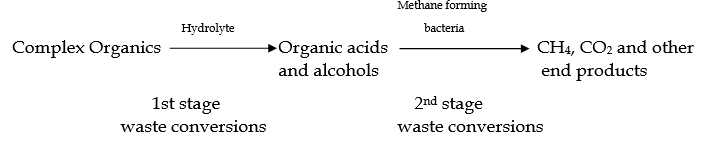
Among available anaerobic processes, Up-flow Anaerobic Sludge Bed or Blanket reactor process is the most important process.
Up-flow Anaerobic Sludge Blanket reactor
Up-flow Anaerobic Sludge Bed (UASB) process is based on the settling ability of microbial flocs to produce a region at the bottom of the digestor where very high biomass concentration can be maintained. The effluent is fed in the bottom of the UASB into the base of the sludge bed. The size of the flocs varies from 1-5 mm in diameter and should have good settling characteristics.
Anaerobic wastewater treatment is preferred to other processes for the following reasons.
- It is cheaper than the aerobic process in respect of the treatment of medium and high-strength wastewaters (COD=1500mg/l).
- Biogas energy is produced instead of wasted.
- Less space is required by the anaerobic plants.
- Low-cost technology in terms of equipment
Thus Upflow Anaerobic Sludge Blanket (UASB) process can be treated as a very promising environmental protection and resource recovery concept.

Primary treatment of wastewater
Primary treatment is a mechanical process involving the removal of organic material that is responsible for 25-30% of the BOD of the sewage. Primary treatment is carried out in a number of steps which are as follows.
a. Screening
Large quantities of floating objects like Cans, Cloths, Wood, etc present in wastewater are usually removed through screens. Different types of screens are available which include bar screens, hand-raked or mechanical raked screens, drum screens,s and wire rope screens. Sometimes instead of screening, the large-sized objects are grounded with the help of a circular grinder called Communicator to a fine size that is removed later in a settling tank.
b. Grit Removal
The grit chamber generally removes inorganic grit which mainly consists of Sand, pebbles, gravel, and cinders. In the grit chamber, the inorganic grit is allowed to settle at the bottom of the chamber which is later on disposed of by use for land-filling, road-making, and in a sludge-drying bed. If the wastewater contains appreciable quantities of oil and grease then it is removed by passing the wastewater through skimming tanks where oil and grease are skimmed off.
c. Sedimentation Removal
Even after the removal of grit, the effluent still has suspended solids which will settle out in a sedimentation tank. In the sedimentation tank, the smaller and lighter particles will settle under gravity. Depending upon the type of settling process, it is termed discrete, flocculent, or zone settling. The most common equipment used includes horizontal flow sedimentation tanks and center-feed circular clarifiers. The settled sludge called raw sludge is removed from the sedimentation tanks by mechanical scraping into the hopper and pumping it out subsequently.
d. Destroying Bacteria
After the removal of larger objects, grit, and sludge, the effluent is treated with chlorine gas to destroy disease-causing bacteria.
Secondary treatment of wastewater
The secondary treatment process is designed to remove most of the remaining organic matter. By this process, about 90-95% of the BOD and bacteria are removed along with 10% of the phosphate and 50% of total nitrogen. Secondary treatment is done by several methods such as i) the Activated sludge process and ii) Trickling filters.
a. Activated sludge process
In this process, the sewage or industrial wastewater is aerated in a reaction tank in which some microbial floc is suspended. The microorganism comes in contact with the dissolved organics and adsorbs them rapidly on their surface. The organic compounds are oxidized to CO2, H2O, and more microorganisms are produced.
b. Trickling Filters:
Trickling filters consist of a filter bed made of rock, gravel, slag, sand, redwood, plastic, and other synthetic materials on which biological slimes are embedded. The wastewater is allowed to percolate through this bed when the organic matter present in wastewater gets adsorbed on slimes and undergoes decomposition by bacteria and fungi present in the slimes. When the slime layers become very thick they slough off and can be easily removed. After the treatment, the water may be chlorinated to kill disease-causing bacteria.
Tertiary Treatment of wastewater
Tertiary treatment is the final treatment meant for polishing the effluent from the secondary treatment processes. Water leaving the secondary treatment is still having,
- Fine suspended solids
- Organic compounds
- Nitrates and Phosphates
- Dissolved inorganic solids
- Diseases causing bacteria and viruses.
These pollutants have to be removed before the effluent is discharged into the water body or into the municipal sewage system. The various pollutants are removed as follows,
- Fine suspended solids
Finely suspended solids can be removed with the help of micro strainers and sand filters.
2. Organic compounds
About 70-80% of Organic compounds from the wastewater are removed by using activated carbon. Activated carbon not only removes organic but also inorganic substances.
3. Nitrates and Phosphates
a. In the secondary treatment, Nitrogen present in the wastewater is converted to nitrates. Nitrobacter can be added in the secondary treatment. The Nitrates so produced can be reduced by anaerobic bacteria to Nitrogen (N2). The process is called denitrification.
b. Phosphorous can be removed by adding lime [Ca(OH)2] and alum [Al2(SO4)3]. The metal cations Ca2+ and Al3+ will react with phosphate to form insoluble phosphate that settles.
4. Dissolved inorganic solids
i) Synthetic or natural ion exchangers can be used for the removal of inorganic materials in the ionic state.
ii) Electrodialysis is electrolysis with a permeable membrane made up of chemically treated plastic which can permit either cations or anions through it. The organic molecules will not be removed and will get accumulated on one side of the membrane. iii) Reverse osmosis, is used in separating water from the wastewater rather than removing waste from water. In reverse osmosis, it is the water and not the ions that pass through the semi-permeable membrane. It reduces both organic and inorganic matter present in wastewater.
5. Diseases causing bacteria and viruses
After the treatment, the water may be chlorinated to kill diseases causing bacteria and viruses.
Coagulation and Flocculation
What are coagulation and flocculation in wastewater treatment? what is the difference between coagulation and flocculation?
a) Coagulation
- Raw water may contain suspended or colloidal particles and bacteria that are too small to settle and therefore cannot be removed by filtration. In such cases, coagulation is the most effective and economical method to remove impurities.
- Most of the colloids remain suspended in solution because they have a net negative charge that causes the particles to repel each other. The charge of colloidal particles can be removed by the addition of a coagulant. This process is known as coagulation. The addition of coagulant neutralizes the colloidal charge, allowing the particles to come together to form larger particles that are easily removed by settling and filtration.
- The common coagulant used is Alum, [Al2(SO4)3]. 18H2O, though FeSO4, FeCl3, and other coagulants such as polyelectrolyte can also be used.
- Alum ionizes in water producing Al3+ ions, some of which neutralize the negative charge on the colloids. Most of the Al3+ ions react with bicarbonates to form insoluble Al(OH)3. The Al(OH)3 absorbs ions from the solution to form a precipitate of Al(OH)3 and adsorbed sulphates.
Al2(SO4)2 18H2O + 6HCO3– —————> 2Al(OH)3 ¯ + 6CO2 + 18H2O + 3SO42-
If sufficient bicarbonates are not available, then the pH must be raised by adding Ca(OH)2 or NaHCO3.
b) Flocculation
Flocculation seeks to achieve agglomeration of small particles into larger particles thereby speeding up the settling process. The most widely used flocculent agent is Alum and salt of aluminum. When Al2(SO4)3 is added to an alkaline solution, a voluminous Al(OH)3 flocculate is produced which readily sinks to the bottom as it is heavier than water. In its movement downwards it traps tiny suspended particles in the water and thus removes them too. Alum is expensive and may leave a carryover of fine turbidity, hence special synthetic polymers like polyacrylamides are used.
Use of Polymer flocculants for industrial waste
When oil is present in the emulsified waste in water, alum and lime are added first to break the emulsion, followed by the polymer. Hot mill wastes could be gravity settled in scale pots, but the effluent from these contains particles between 2-70 microns in diameter. With a feed of 0.3 mg/liter of anionic polyacrylamide, the suspended solids can be reduced to about 40 mg/liter. For oil refinery waste liquors, a combination of 25-100 ppm of alum followed by 1-3 ppm of polyamine has been used and it produces a dense and large flocculate which settles down easily.
Please also read about Gas Chromatography
What is the difference between BOD and COD?
Biological oxygen demand indicates “the amount of O2 required for biological oxidation of organic matter present in wastewater by microorganisms” and Chemical Oxygen Demand is “the measure of the amount of O2 required for oxidation of organic matter present in wastewater by strong chemical oxidants like K2Cr2O7 or KMnO4
What is the full form of BOD?
Biological Oxygen Demand.
What is the full form of COD
Chemical Oxygen Demand.
What is the chemical oxygen demand formula?
(V1-V2) x N x 8 x 1000 / X
What is BOD and why is it important?
It is an important parameter for assessing the quality of water.
What happens if BOD is high?
Due to high BOD the aquatic organisms may become stressed, suffocate, and can die.
Hi there! Would you mind if I share your blog with my twitter group? There’s a lot of people that I think would really appreciate your content. Please let me know. Thank you
Sure.. why not?
Thank you very much, dear…..
I have been reading out many of your posts and i can state nice stuff. I will surely bookmark your blog.
Thank you very much dear…..
I抳e been exploring for a little for any high quality articles or blog posts on this sort of area . Exploring in Yahoo I at last stumbled upon this web site. Reading this info So i抦 happy to convey that I’ve a very good uncanny feeling I discovered just what I needed. I most certainly will make certain to don抰 forget this website and give it a look on a constant basis.
Thank you very much…
I¦ve read a few excellent stuff here. Certainly price bookmarking for revisiting. I wonder how much effort you set to make this sort of great informative web site.
Thank you very much…
Thanks for the sensible critique. Me and my neighbor were just preparing to do a little research about this. We got a grab a book from our local library but I think I learned more clear from this post. I am very glad to see such great info being shared freely out there.
Thank you very much…
I like this web site very much, Its a real nice place to read and find info . “Never contend with a man who has nothing to lose.” by Baltasar Gracian.
Thank you