The accuracy of a single measurement or that of sets of measurements is inversely related to the magnitude of error involved. Error is defined as the departure of the measured value from its true value. In terms of precision’ is a measure of the degree of reproducibility of experimental result.
Any analytical method in the context of analytical chemistry will involve the measurement. Measurements by methods that give results that are reliable, reproducible, and accurate. But no matter how carefully and systematically an observation is recorded, no measurement is free from error and unfortunately the magnitude of error is difficult to quantify. This is because the magnitude of error is the difference between the measured value and the true value.
True value is an elusive philosophical quantity that may or may not be known exactly so one can only try to decrease the margin of error to make the result sufficiently accurate to an acceptable level. Error and accuracy are relative terms and can have different meanings when used in different contexts. So it is necessary to understand the theory of error and its sources to obtain the best possible result.
Error is classified into 1) Indeterminate Error and
2) Determinate Error (as per their origin)
Indeterminate Error : ( Random Error)
i) Indeterminate errors are those whose causes can be not easily located or whose source of error cannot be pinpointed.
ii) These errors are of a fluctuating and random type and hence are also called ‘random errors’. They will scatter the observations on both sides of the ‘True Value’ i.e. observations greater than and less than the true value is of equal probability.
iii) They are of small magnitude, because of which the mean of the set of observations is not affected which finally does not affect the result of analysis.
iv) The more elaborate in procedure and the more the number of steps involved higher the chances of introducing random error.
vi) Statistical methods of analysis are used to study the effects of indeterminate errors on the final result.
Determinate Error : ( Systematic Error)
i) Determinate errors are errors that can be measured and accounted for, a least in principle, and to which definite measurable value can be ascribed.
ii) Determinate errors are generally unidirectional concerning the true value and are of considerable magnitude as a result they can distort the final result to an extent.
iii) Determinate errors are reproducible and to a certain extent, in some cases, predictable as well hence are also called systematic errors.
v) By an appropriate choice of equipment, apparatus, and method of analysis. Systematic errors can be eliminated.
vi) Determinate error can be further classified into instrumental methodic operational & personal error.
Distinguish between Determinate and indeterminate error
| Determinate or Systematic error | Indeterminate or Random error | ||
| 1) | For these types of errors, the source can be traced. | 1) | For these types of errors, the source cannot be traced. |
| 2) | Determinate errors are of larger magnitude. | 2) | Indeterminate errors are of smaller magnitude. |
| 3) | Determinate error of only positive or negative type. i.e. unidirectional. | 3) | Elementary irregularities are responsible for random errors. |
| 4) | They can be eliminated by proper precaution | 4) | They can be only minimized not eliminated. |
| 5) | Elementary irregularities are responsible for random errors. | 5) | Elementary irregularities are responsible for random error. |
Classification / various types of determinate errors
Determinate error is classified into the following types.
1) Instrumental error, 2) Methodic Error, 3) Operational error, 4) Personal error
Instrumental Error
Irrespective of the method of analysis, at some stages, the use of the instrument or an apparatus forms an integral part of the analysis, which can lead to the introduction of error through the source of the instrument. Instrumental error may be either due to the instrument itself or due to the effect of environmental factors on the instrument.
Causes of Instrumental Error
Uncertainty in an observation, recorded with the help of an instrument, every instrument possesses a definite least count which can be the cause of instrumental error. (Eg:- If the burette reading is 15.38 cm3 or 15.43 cm3 but the burette used has the least count of 0.1 cm3, then it will read both the above readings as 15.4 cm3)
Every instrument has its optimum range of working within which readings obtained are perfect, but outside this range, readings can be improper or errorful. (Eg:- a pH meter designed to measure from 1 to 10 will work optimum in range 1 to 10, Outside this range reading will be shown by the pH meter, but can be an improver)
Faulty balance or weight machine.
Poorly calibrated glassware and instruments.
Drift in the electronic circuits.
Decrease in voltage of batteries used.
The corrosive action of reagents on glassware leads to errors.
Methodic Error
Methodic errors are those errors that are part and parcel of a given method of analysis and will get transferred to an observation due to the use of the method. Since they are part of the method they can be only minimized but cannot be eliminated,
Examples of methodic errors
Solubility of a Salt.
The gravimetric analysis involves the precipitation of salt and its separation, but the solution that is left behind is saturated with salt hence complete separation by precipitation is not possible.
Addition of excess amount of titrant
The completion of the reaction in the volumetric analysis is indicated by a change in colour of the indicator used. This colour change occurs only when an extra amount of titrant (burette solution) is added, Hence titration always requires more volume of titrant than required for the completion of the reaction.
Incomplete reaction
In particular analysis, the reaction may not go to completion and hence causes the error in measurement of a heterocyclic aromatic compound containing nitrogen as part of the ring system, which does not get completely oxidized by digestion with hot conc. H2SO4.
Co-precipitation and post-precipitation
Both phenomena are observed in gravimetric analysis. This is because the precipitate in contact with the solution may absorb ions from it on its surface.
Incomplete decomposition
Iron analysis by gravimetry involves precipitation as Fe(OH)3 and is then decomposed to Fe2O3 and weighed. If not decomposed properly can lead to error.
Operational Error
Errors that are introduced in the execution of a method of analysis are classified as operational errors. The reason for the introduction of this error is the person carrying the analysis that is the analyst. It arises due to insufficient knowledge or total ignorance of handling a piece of equipment and not taking the necessary precautions in measurement. A few examples,
a. Lack of experience of analysts resulting in errors in weighing and volume readings.
b. Not covering the sample container leads to the introduction of foreign materials in the sample.
c. Weighing a crucible when it is in hot condition. Cooling the crucible in desiccators with poor desiccant.
d. Underwashing or overwashing precipitate. Drying or Ignition of precipitate at incorrect temperature.
e. Error in calculations. Operational error can be avoided.
Personal Errors or Human Errors
Personal errors arise due to faulty ideas, bias, and physical limitations of the analyst.
For instances,
a. Blowing the pipette or warming the bulb of the pipette by palm to remove the so-called ‘Last Drop’ leads to the addition of an extra drop
b. The experimenter may have a bias or prejudice under which he always tries to match his next reading with the readings that he obtained earlier, just to maintain precision.
c. Poor eyesight and colour blindness are examples of physical limitations.
Methods adopted to Minimize determinate types of errors
Once the source of determinate error is located, proper measures can be adopted to minimize the errors to an acceptable level.
Steps considered to minimize the determinate errors,
Calibration of the Apparatus
All the apparatus like burettes, pipettes, standard flasks, and weights should be calibrated. All instruments are calibrated at a certain temperature, at which they should be used. If it is not possible then temperature correction should be applied. In this way, instrumental error can be minimized
Running control determination
In control determination, a standard sample with exactly known value (i.e. true value) is subjected to a method of analysis then the result obtained is compared. The difference between the obtained value and the true value of the standard sample can be taken as a measure of both personal and methodic error and can be applied for correction to minimize error.
Running of blank determination
Blank determination involves repeating the analysis without the sample under study under identical conditions and in the same procedure, the difference obtained is applied as a correction to actual measurement it helps to minimize methodic & operational error.
Use of an independent method of analysis
In some cases, there may be more than one method of analysis for a particular sample. Thus, the same sample can be analyzed by two different methods of analysis and a comparison of the results obtained can be made. As the two methods are different, methodic errors in the two cases will also be different, hence this case be used to determine the proper method of analysis.
Running parallel determination
In this case, the same sample is analyzed by two different analyzed by the same methods or by the same analyst, and different methods of replicate determination will improve the reproducibility or precision of the set.
Standard addition method
In this method initially, the component of the sample is analyzed then a known concentration of the same component is added to the sample and now a reading of this mixture is taken.
The difference in results for samples with and without the component is evaluated. The process can be repeated by increasing the volume of standard solution added. The result obtained can be used to calculate the amount of components in the sample.
Amplification method
If the concentration of the sample under study is low then the magnitude of the response given by the sample will also be small. Measurement of small quantities will automatically involve more uncertainty and hence more magnitude of error. Hence by adding the same sample component in a larger volume to the sample response can be amplified to reduce the uncertainty and error involved.
Training and experience
Personal error can be minimized to an acceptable level by way of training, experience, self-discipline, and experimenting with great care. Rechecking instrumental readings and calculations helps to minimize personal errors.
MEASURES OF ERRORS
Absolute error (A.E.)
It is defined by the difference between the measured value and the true or accepted value.
Absolute error = x i – T
Where, x i = Measured value & T = True Value
Relative Error (R.E.)
It is defined as the ratio of absolute error to the true value. It is expressed as,
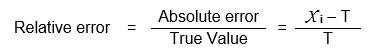
R.E. is expressed in either parts per thousand (ppt) or parts per hundred (pph)
Constant and Proportionate Errors
Constant Errors
Constant error is defined as an error in which the absolute error is independent of sample size, but the relative error is inversely proportional to sample size i.e. In constant error absolute error remains unaffected with a change in sample weight but, the relative error increases if the sample weight is decreased and relative error will decrease if the sample weight is increased.
For example :
In titrimetric analysis, the indicator is used to show the completion of the reaction by giving a colour change. Colour change is always shown up when an extra drop of burette solution is added. As shown in the table below different samples of different titer values 50, 10, & 1 cm3 are taken all the samples will require that ‘0.1 cm3’ of the extra drop of burette solution to show the colour change, hence 0.1 cm3 will form the absolute error in all the case of the sample. Further by taking titer values 50, 10, and 1 cm3 as True values (respectively), the relative error is calculated.
| Sample No. | True Value (cm3) | Absolute Error (cm3) | Relative Error (cm3) |
| 1 | 50 | 0.1 | 0.2 |
| 2 | 10 | 0.1 | 1 |
| 3 | 01 | 0.1 | 10 |
So above table gives an example of constant error since with a decrease in sample size (titer value), absolute error is constant but relative error goes on increasing.
Proportionate Error–
It is defined as an error in which the absolute error is directly proportional to sample size, but the relative error is independent of sample size i.e. Absolute error increase of sample size increases and decreases if sample size decreases but in both cases, the relative error remains constant.
For Example
Consider the determination of Lead which is contaminated by barium on impurity lead is precipitated on PbSO4 but along the lead, barium is also ppt as BaSO4 whose weight will depend on the volume of the original solution used for precipitation the following table illustrates proportionate error since BaSO4 is contaminated its weight forms absolute error, while the weight of PbSO4 is the true value.
| Sample Size | Amount of PbSO4 +BaSO4 | Amount of BaSO4 | Absolute error | Relative error (pph) |
| 0.5 g | 100 mg | 2 mg | 2 mg | 2 % |
| 1.0 g | 200 mg | 4 mg | 4 mg | 2% |
| 0.25 g | 50 mg | 1 mg | 1 mg | 2% |
As seen in the table in proportionate error. Absolute error is directly proportional to sample size while relative error is constant.
Distinguish / Difference between constant and proportionate error.
| Constant Error | Proportionate Error | ||
| 1 | Constant errors are those in which magnitude of absolute error is constant. | 1 | Constant errors are those in which the magnitude of absolute error is constant. |
| 2 | Relative error decreases with an increase in sample size | 2 | Relative error is constant |
| 3 | The sample size should be as large as possible for good results. | 3 | In proportionate error, the magnitude of absolute error keeps changing. |
Accuracy and Precision
Accuracy
The accuracy of a measurement is defined as the closeness of the observed value to the true value.
The accuracy of a single measurement or that of sets of measurements is inversely related to the magnitude of error involved.
Greater accuracy indicates a low magnitude of error.
Accuracy is a measure of the reliability of a particular measurement.
Accuracy is measured in terms of Absolute and relative error
Precision
In terms of precision’ is a measure of the degree of reproducibility of experimental result. It can be defined as the agreement between two or more values of the same sets of observations, studied under identical conditions.
A result is said to be highly precise when most of the values obtained are the same but ‘Precision’ is not a guarantee of accuracy.
Precision is calculated in terms of deviations.
The precision of a single measured value cannot be calculated, it can be calculated for sets of readings, obtained under identical conditions.

Difference / Distinguish between Accuracy and precision
| Accuracy | Precision | ||
| 1) | Accuracy stands for the reliability of a measurement. | 1) | It is the agreement amongst the observation of a set. |
| 2) | Precision is measured in terms of average deviation. | 2) | It stands for the reproducibility of measurement. |
| 3) | Accuracy is never calculated exactly since the true value of any entity is never exactly known. | 3) | Accuracy is expressed in terms of absolute and relative error. |
| 4) | Accuracy for a single value can be calculated if the true value is known. | 4) | Even if the true value is known then precision of a single value cannot be calculated. |
| 5) | Even if the true value is known the precision of a single value cannot be calculated. | 5) | The precision of a set of observations can be evaluated by repeating the sets of observations. |
Some important and Useful Articles –
Thermogravimetric Analysis TGA principle, instrumentation | Application of thermogravimetric analysis
Thermal Analysis is a method in which physical or chemical properties of a sample (pure substance or mixture of substance and/or reaction mixture) are measured as a function of temperature or time, by the sample is subjected to a control temperature program… Read more
Molecular fluorescence and phosphorescence spectroscopy | Fluorimetry and Phosphorimetry – instrumentation, and applications |
An excited molecule comes back to the ground state by emitting an absorbed light in the form of UV light or visible light photon. This process is called Fluorescence… Read more
FAQs
Define Error or What is error?
Error is defined as the departure of the measured value from its true value
What is Proportionate Error?
Constant errors are those in which the magnitude of absolute error is constant.
What is Precision?
Precision is the agreement amongst the observation of a set.