Optical Method of analysis | Basics of Atomic emission spectroscopy – Flame emission spectroscopy (Flame photometry)
Optical methods of analysis are non-destructive techniques for testing. Optical methods for analysis are based on either absorbance or fluorescence.
There are two main types of Atomic spectroscopy namely Atomic emission spectroscopy- Flame emission spectroscopy (Flame photometry) and Atomic absorption spectroscopy (AAS).
Atomic Emission Spectroscopy (AES)
When an element is heated to a high temperature in a flame, it is converted into atoms. A few of its atoms absorb thermal energy from flame and get excited. This excitation is due to the transition of the atom from a ground energy level to a higher energy level. The heat required for the transition is provided by flame temperature or electric arc or spark.
The excitation of an atom is quickly followed by the emission of radiation and the return of the atom to the ground state. The energy radiated is given by hv= E2 – E1. The emitted radiation is made up of many frequencies, these emitted frequencies produce distinct lines when observed in the spectrometer.
These lines are characteristic of an element and serve as the identity of the element, this constitutes the atomic emission spectrum of the element under study.
Flame Photometry or Flame Emission Spectroscopy (FES)
It involves the study of the emission of radiation by atoms when their salt solution is introduced into the flame. When in flame, atoms get excited from the lower higher excited state, such an excited atom emits energy while coming back to the ground state in the form of radiation. Measurement of this radiant energy forms the basis of FES.
If the emitted radiation is observed in the spectrometer, a line constituting the emission spectrum characteristic to the particular atom is obtained. The intensity of the lines in the emission spectrum of an atom is a function of concentration.
Principle of Flame Photometry or Flame Emission Spectroscopy
FES is the study of emission radiation by atoms when their salt solution is introduced into flame.
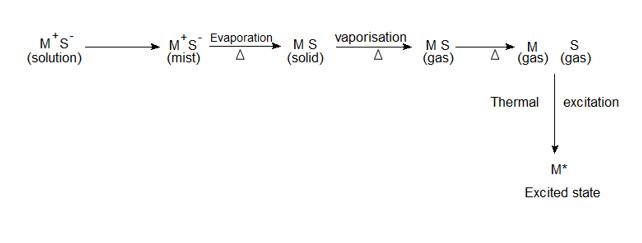
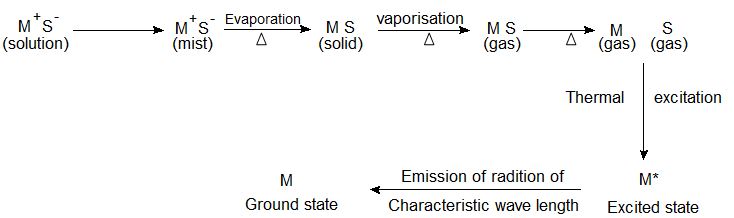
When a liquid sample containing a metallic salt solution MS is introduced into flame, the processes involved here are complex are simplified as follows:
The solvent evaporates leaving the solid MS.
The solid is dissociated by vaporization into the gaseous atom of M & S.
The atom of a metal (M) is raised to a higher energy level by the heat of the flame.
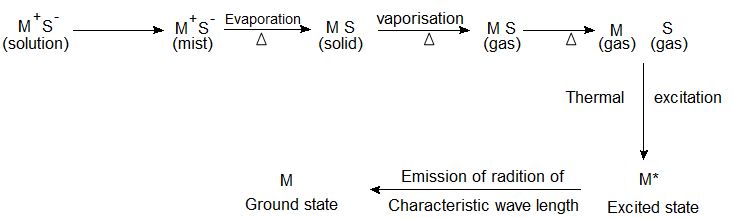
The excited atoms emit energy in the form of radiation. This sequence of atomization is schematically shown above.
If E0 is the ground state energy and E1 the excited energy then the frequency of radiation emitted when the atom falls back to the ground state can be given as:
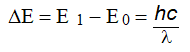
It is possible to calculate the wavelength of emitted radiation which is characteristic of an atom under study. The wavelength of emitted radiation is used for qualitative analysis i.e identification of the element under study and the intensity of light emitted is used for calculating the amount i.e the concentration of the element under study.
The number of atoms excited in flame is a small fraction of the total atom population. The fraction of the total thermal excited atoms can be given by the Boltzmann distribution equation.
Instrumentation of Flame photometry or Emission Spectroscopy

Burner (Atomizer)
Burners are actually Nebuliser-burner system that converts the liquid sample into fine droplets and introduce it in flame where the atomization of the sample takes place which finally results in the excitation of the atom followed by the emission of radiation.
Flame temperature is an important parameter in this hence the flame temperature should lie between 10000C to 30000C hence fuel-oxidant gas mixture is employed they are as follows:
| Fuel gas | Oxidizer gas | Temperature 0C |
| Acetylene | Air | 2200-2350 |
| Hydrogen | Oxygen | 2125-2400 |
| Acetylene | Oxygen | 2550-3135 |
| Acetylene | Nitrogen oxide | 2600-2800 |
| Hydrogen | Air | 2000-2050 |
Two types of burners are used in these instruments
Total Consumption Burner
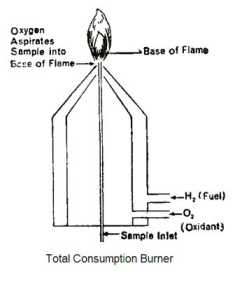
The Total consumption Burner is such a burner where there is complete consumption of fuel. It is made up of three concentric tubes. The middle tube is a fine tube from where the sample solution is sprayed directly into the flame. The other two adjacent tube carries fuel and an oxidizer. All three component mix at the tip of the burner. The sample solution rises upward due to the vacuum created on the top of the tube. At the tip gas mix with oxidizer and burns. It is also known as the nebulization process.
Advantages of The Total Consumption Burner
a. In the total consumption burner entire sample is used for analysis, (hence the name total consumption burner).
b. It is very sensitive.
c. Ratio of fuel: Oxidant can be adjusted; hence temp of flame can be adjusted.
Limitation of The Total Consumption Burner
a. Uniform droplets of the sample is not formed, which leads to fluctuation of emission intensity.
b. The flame is noisy and turbulent.
Pre-mix or Laminar flow Burner
Laminar means smooth. Pre-mix or Laminar flow burner is a type of burner specially designed for a stable, nonturbulent flame. In the Laminar flow burner, the fuel and air are carefully mixed to produce a uniform mixture that flows smoothly through the burner.

Laminar flow burners are generally used where the stable or non-turbulent flame is important.
In this the fuel, oxidant, and sample under study are thoroughly mixed before entering the flame, hence the name pre-mix.
Advantages of Laminar flow burner
a. Nonnoisy.
b. Non turbulent flame.
c. Efficient atomization.
Limitation of Laminar flow burner
a. Only 5% of the sample is used.
b. Since a small amount of sample is used intensity is Weaker.
Mirror
The light from the flame is reflected by a concave mirror placed behind the burner. The reflected beam is then focused onto the filter.
Monochromator
The filter allows the characteristic radiation of the element to pass through and will absorb all other unwanted radiations. If the reflected beam consists of a large number of wavelengths, a monochromator (Grating or Prism) is used to disperse the reflected beam into its individual wavelengths. When a monochromator is rotated, different wavelengths are focused one by one on the photocell. This scanning enables us to detect several elements simultaneously.
Photocell or Photomultiplier Tube
The role of the Photocell or Photomultiplier Tube is to amplify and read the electric current received from the monochromator.
Detector
To amplify and read.
Working of flame photometer
- Using a Blank solution instrument is first adjusted to zero-emission
- The sample is then drawn into an atomizer (i.e burner) by suction.
- The sample solution is converted into mist, which gets mixed with fuel and oxidizer gas
- This sample mixture burns at the burner head.
- Some of the atoms of the sample go to an excited state and emit characteristic radiation while coming back to the ground state.
- Light emitted passes through the monochromator which selects the characteristic radiation of the sample under study only.
Selected radiation falls on the photocell and output is measured and displayed.
Application of Flame photometry or Flame Emission Spectroscopy
Flame Emission Spectroscopy is an effective tool for the detection of alkali and alkali earth metals. It is widely used for the estimation of Na, K & Ca in blood serum, urine, soil extraction, and water sample.
Determination of Ca & Mg in cement.
Determination of sodium in the soil by flame photometry.
Determination of lead in petrol.
Simultaneous determination of Na & K from the biological product, foods, fertilizers, etc.
The presence of Na, K, Li, Ca, & Mg in glass can be estimated.
Equilibrium constants involving ion exchange resins can be studied.
In clinical analysis and physiology, the electrolyte balance can be studied.
Limitations of Flame Photometry or Flame emission spectroscopy
Flame emission spectroscopy is not applicable to all metals.
Detection and estimation of inert gases can not be possible.
Provides results in solutions only and not directly with solid samples.
The elements like carbon, hydrogen, and halides cannot be detected due to their non-radiating nature.
The accurate concentration of the metal ion in the solution cannot be measured.
FAQs
What is flame photometry? or What is the principle of flame photometry?
Flame photometry involves the study of the emission of radiation by atoms when their salt solution is introduced into the flame. When in flame, atoms get excited from the lower higher excited state, such an excited atom emits energy while coming back to the ground state in the form of radiation. Measurement of this radiant energy forms the basis of FES.
What is photometry?
In chemistry, photometry refers to the measurement of the intensity of light that is absorbed or transmitted by a sample in order to determine the concentration of a particular substance in the sample.
What is the difference between atomic absorption spectroscopy and flame emission?
In flame emission spectroscopy, certain wavelengths of light emitted from atoms are recorded, whereas during atomic absorption spectroscopy, certain wavelengths of light are absorbed are recorded.
One thought on “Optical Method of analysis | Basics of Atomic emission spectroscopy – Flame emission spectroscopy (Flame photometry)”